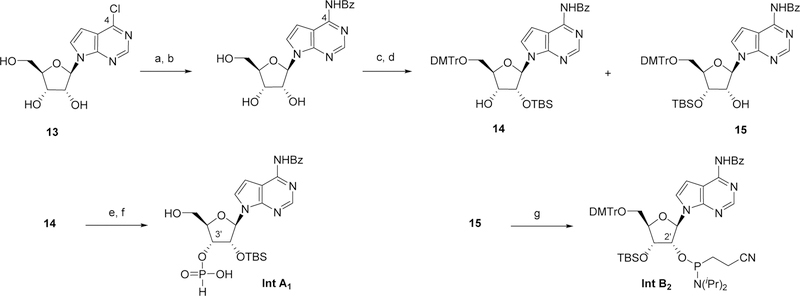
Scheme 2.
Reaction conditions: a) NH3, MeOH, 110 °C; b) i. TMSCl, pyridine, 0 °C; ii. BzCl, 0 °C to RT; iii. H2O, then aq. NH3 0 °C; c) DMTrCl, pyridine; d) TBSCl, pyridine, AgNO3; e) PivCl, pyridine, H3PO3; f) Cl2CHCO2H, H2O, DCM; g) 2-cyanoethyl diisopropylchlorophosphoramidite, DCI, DCM. Bz = benzoate; DMTr = 4,4-Dimethoxytrityl; TBS = tert-Butyldimethylsilyl.