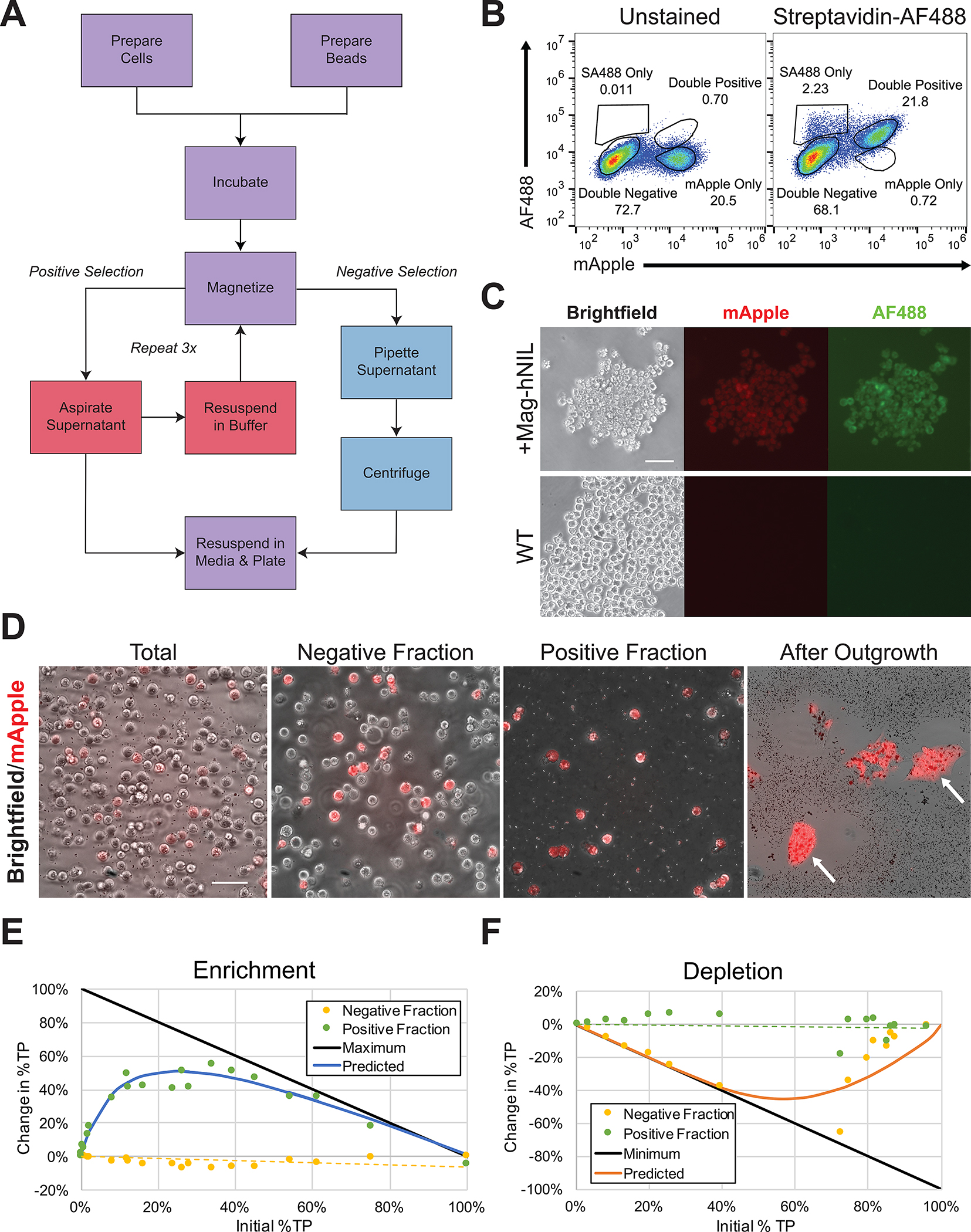
Figure 2. Enrichment of transgenic iPSCs by magnetic streptavidin bead affinity.
A. Workflow of positive and negative selection protocols. See protocol for considerations on key steps, such as cell number, bead volume, and incubation time.
B. A mixed population of unedited cells and cells transfected with the Mag-hNIL construct and of unedited cells were incubated with streptavidin conjugated to AlexaFluor 488 and washed with PBS once. Staining is mostly specific for edited cells, and mApple is strongly co-expressed with SBP-LNGFR.
C. Pure populations of cells with integrated Mag-hNIL or of unedited cells were incubated with streptavidin conjugated to AlexaFluor 488 and washed with PBS once before fluorescent imaging to confirm co-expression and specificity of streptavidin binding.
D. A mixed population of cells was dissociated by EDTA, and the total, negative, and positive fractions were imaged and quantified. The total fraction contains 21% mApple-expressing cells, the negative fraction contains 17%, and the positive fraction contains 97%. The positive fraction was then plated, and multiple pure clones were observed following outgrowth.
E. Pure populations of true positive (TP) or true negative (TN) cells were mixed at various ratios, and the enrichment protocol was performed as suggested. Total, negative, and positive fractions were collected from each sample and quantified for %TP by flow cytometry for mApple expression. Data shown is the difference between the total fraction and positive or negative fraction %TP, and maximum represents a pure positive fraction. Enrichment is highly predictable as a function of the initial %TP, with optimal gains in initial populations of 10–50%TP, often resulting in positive fractions >90%TP. It is also especially effective for rare populations in proportion to their initial %TP, resulting in up to 10-fold increases.
F. The experiment from E was performed using the depletion protocol as suggested, and minimum represents a pure negative fraction. A similar predictable relationship was observed between initial %TP and reduction in the %TP of the negative fraction was observed, although low levels of cell recovery in high initial %TP samples resulted in increased variability and low cell recovery was observed at high initial %TP.