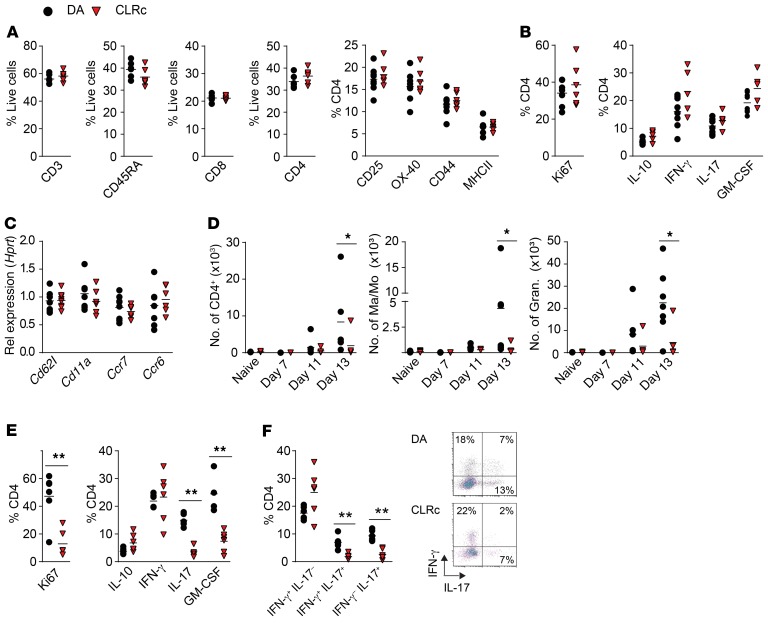
Figure 2. Modulation of T cell activation in the CNS, but not in the periphery, underlies the protective CLRc phenotype.
Homozygous CLRc rats and littermate DA controls were immunized with MOG and phenotyped during the disease course. Characterization of cells infiltrating draining lymph nodes (dLNs) and the CNS. (A) Frequency of total T cells (CD3), B cells (CD45RA), and CD8+ and CD4+ T cells on day 7 p.i. in dLNs of DA (n = 6–8) and CLRc (n = 6) rats. Frequency of activation markers on CD4+ T cells (representative of 4 experiments). (B) Ki67 expression and cytokine production on day 7 p.i. in CD4+ T cells from dLNs of DA (n = 7–8) and CLRc (n = 5–6) rats following 72-hour in vitro MOG stimulation (representative of 3 experiments). (C) qPCR for Cd62l, Cd11a, Ccr7, and Ccr6 in CD4+ T cells sorted from dLNs of DA (n = 6) and CLRc (n = 6) rats on day 7 p.i. (representative of 2 experiments). (D) Number of infiltrating CD4+ T cells, macrophages/monocytes (Ma/Mo), and granulocytes in spinal cord of naive DA and CLRc rats (n = 12 and n = 12) and at 7 (n = 3 and n = 3), 11 (n = 6 and n = 5), and 13 days p.i. (n = 7 and n = 5) (representative of 2 experiments). (E and F) Characterization of proliferation and cytokine production in infiltrating cells isolated from spinal cord on day 13 p.i. stimulated in vitro with PMA/ionomycin/brefeldin A for 5 hours in CLRc rats (n = 6) and DA rats (n = 6) (representative of 2 experiments). Data are presented as the mean ± SEM. All comparisons were analyzed with the Mann-Whitney U test. *P < 0.05; **P < 0.01.