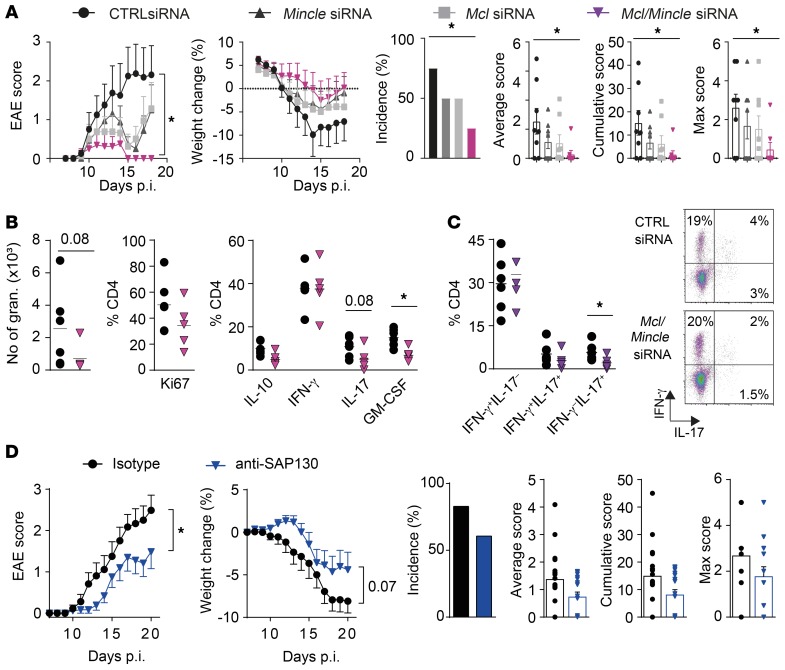
Figure 5. CNS-specific Mcl/Mincle silencing, as well as blockage of the endogenous ligand SAP130, protects from EAE.
(A) MOG-immunized rats injected with Mcl (n = 8), Mincle (n = 8), Mcl/Mincle (n = 8), or scrambled control siRNA (n = 8) i.t. and i.c. at 7, 9, and 12 days p.i. were followed for clinical signs of EAE (representative of 3 experiments). (B) Characterization of proliferation and cytokine production of infiltrating cells isolated from spinal cord on day 12 p.i., stimulated in vitro with PMA/ionomycin/brefeldin A for 5 hours (proliferation and cytokine production) in rats injected with Mcl/Mincle (n = 5) or scrambled control siRNA (n = 6) and (C) quantification of IFN-γ– and IL-17–producing CD4+ T cells (representative of 2 experiments). (D) Clinical signs of EAE and disease parameters in DA rats treated with anti-SAP130 (n = 18) or rabbit IgG isotype control (n = 18) antibody i.t. and i.c. on days 2 and 7 p.i. (2 pooled experiments). Data are presented as the mean ± SEM. The following statistical tests were used: 1-way ANOVA with Dunnett’s multiple-comparisons test (A, for area under the curve [AUC] of clinical EAE and weight change), Mann-Whitney U test (A–D [for average, cumulative, and max EAE score], B, and C), unpaired 2-tailed t test (D, for AUC of clinical EAE and weight change), and χ2 test (A and D, for EAE incidence). *P < 0.05.