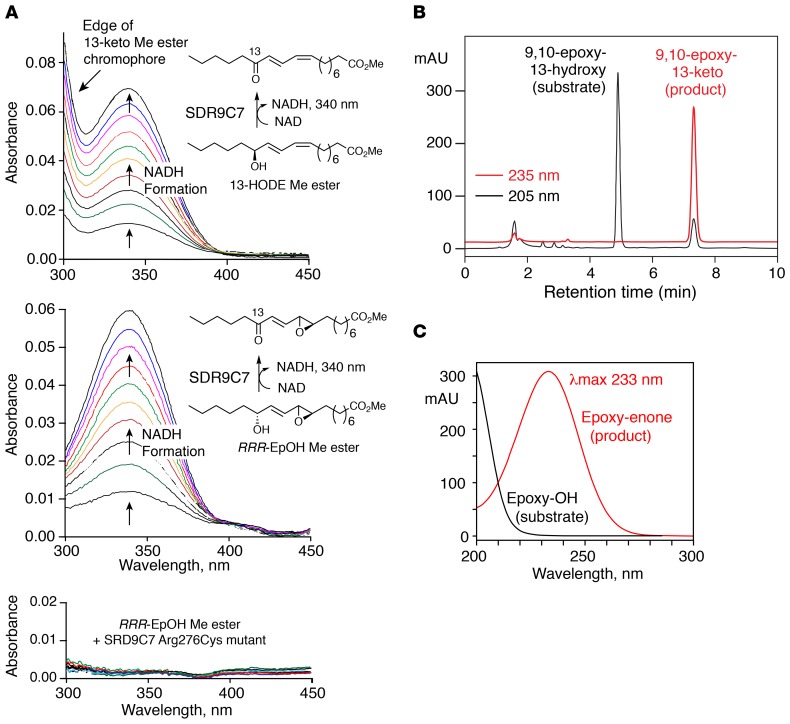
Figure 5. SDR9C7 catalyzes NAD+-dependent oxidation of 13-hydroxy-linoleate derivatives.
(A) Activity of recombinant SDR9C7 monitored by spectrophotometric assay of NADH formation. Top panel: SDR9C7 catalyzes oxidation of 13-HODE methyl ester (13-HODE Me) to the 13-ketone. In addition to the appearance of NADH at 340 nm, the scans in the metabolism of 13-HODE show an increase near 300 nm, which represents the edge of the UV chromophore of the 13-keto oxidation product (λmax at 279 nm); this increase in absorbance is not evident in metabolism of the epoxy-alcohol (middle panel), because its oxidation product absorbs much lower in the UV (λmax 231 nm) (10). Middle panel: SDR9C7 catalyzes oxidation of the epoxy-alcohol 9R,10R-trans-epoxy-11E-13R-hydroxy-octadecenoate methyl ester (RRR-EpOH-Me) to the 13-ketone. Lower panel: the SDR9C7 R276C mutant has no detectable activity in oxidation of RRR-EpOH-Me, (as further confirmed by HPLC analysis of the incubation). (B) Reversed-phase HPLC analysis of the SDR9C7-catalyzed oxidation of RRR-EpOH-Me. The unreacted substrate is detected in the channel monitoring 205 nm, the single product mainly at 235 nm. (C) The 9,10-epoxy-11E-13-keto product was identified from its cochromatography with the authentic standard and by its characteristic UV spectrum. The HPLC analysis used an Agilent 1200 series system with an Agilent Eclipse XBD-C18 column (5 μm, 15 × 0.46 cm), a solvent of acetonitrile/water/glacial acetic acid (75/25/0.01 by volume) at a flow rate of 1 mL/min, with the diode array detector set to monitor at 205, 220, 235, and 270 nm.