Fig. 4. A new N-SH2:C-SH2 interface forms upon simultaneous binding of ITIM-[dPEG4]2-ITSM to both SH2 domains of SHP21–220.
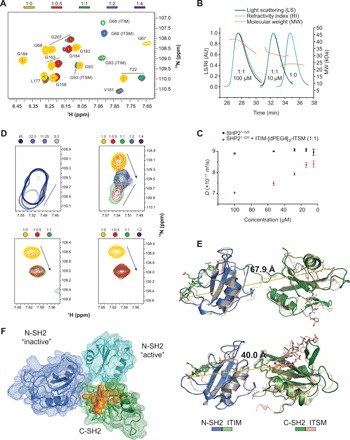
(A) Excerpt from the 1H-15N NMR spectrum of SHP21–220 in the presence of different stoichiometric ratios of ITIM-[dPEG4]2-ITSM shows two sets of peaks for amino acids 1 to 110, matching those of the N-SH2–ITIM and N-SH2–ITSM complexes. With excess peptide, the ITSM-bound peak becomes dominant. Colors represent protein:peptide molar equivalents. (B) Size-exclusion chromatography (SEC)–multiangle light scattering (MALS) profiles of equimolar SHP21–220:ITSM-[dPEG4]2-ITSM mixtures at either 10 or 100 μM, together with the profile of the unbound protein. (C) Translation diffusion coefficients measured by NMR DOSY (diffusion-ordered spectroscopy) for equimolar SHP21–220:ITIM-[dPEG4]2-ITSM mixtures at concentrations in the 5 to 100 μM range. (D) Top left: S189 CSPs upon dilution of a 1:1.2 SHP21–220:ITIM-[dPEG4]2-ITSM mixture. Shades of blue represent different concentrations. Top right: S189 CSPs upon titration of increasing molar ratios of ITIM-[dPEG4]2-ITSM ([SHP21–220] = 100 μM). The straight lines and arrows indicate concentration-dependent CSPs and CSPs from the unbound to the bound state, respectively. The peak indicative of the new interdomain interface reaches its maximum intensity at equimolar SHP21–220:ITIM-[dPEG4]2-ITSM and disappears with excess peptide. Bottom left and right: Titration of a mixture of isolated N-SH2 and C-SH2 at 200 μM each (left) or C-SH2 alone (right) with ITIM-[dPEG4]2-ITSM. The S189 peak corresponding to the new interdomain interface is not visible. (E) Homology models of SHP1–220 with ITIM and ITSM bound to the N-SH2 and C-SH2 domain, respectively, obtained by superposition of the N-SH2–ITIM and C-SH2–ITSM structures on the respective SH2 domains of PDB entry 2SHP (top, autoinhibited state of SHP2) and PDB entry 3PS5 (bottom, open state of SHP1). The dashed lines represent the distance between the ITIM C terminus and the ITSM N terminus. The length of the extended connecting linker in PD-1 is ~40 Å. (F) Superposition of the SHP21–220 models of (E) aligned on the C-SH2 domain (green). The N-SH2 domains from PDB entries 2SHP and 3PS5 are in blue and cyan, respectively. Residues in orange show CSPs upon dilution of the equimolar SHP21–220:ITIM-[dPEG4]2-ITSM mixture (D) and are located at the N-SH2:C-SH2 interface in either structure.
