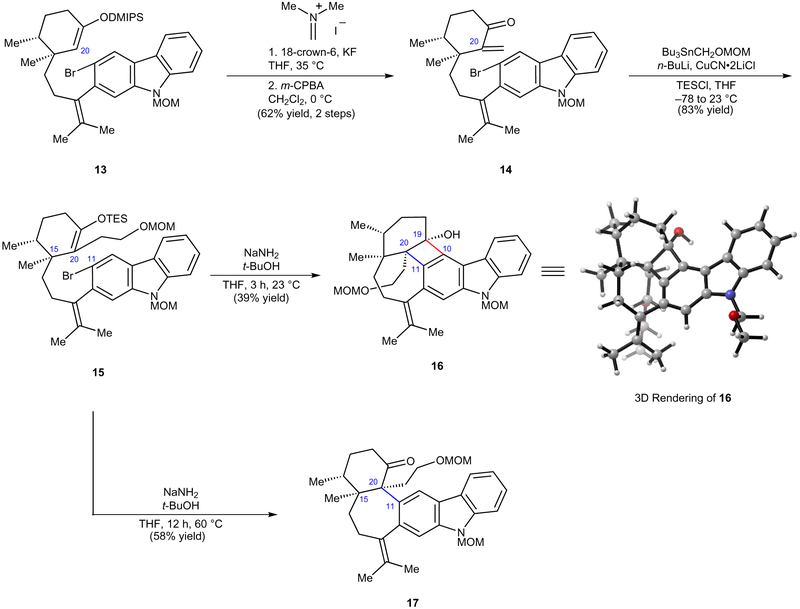
Figure 4 |. The key aryne cyclization forges the C20–C11 linkage.
Enol ether 15 was synthesized in three steps from 13 and assessed in the critical carbazolyne cyclization. Although the use of conventional aryne cyclization conditions delivered the undesired cyclobutenol 16, switching to longer reaction times and higher temperatures furnished the desired pentacyclic ketone 17. 18-crown-6, 1,4,7,10,13,16-hexaoxacyclooctadecane; MOM, methoxymethyl; DMIPS, dimethyl(isopropyl)silyl; THF, tetrahydrofuran; m-CPBA, meta-chloroperbenzoic acid; TESCl, triethylsilyl chloride.