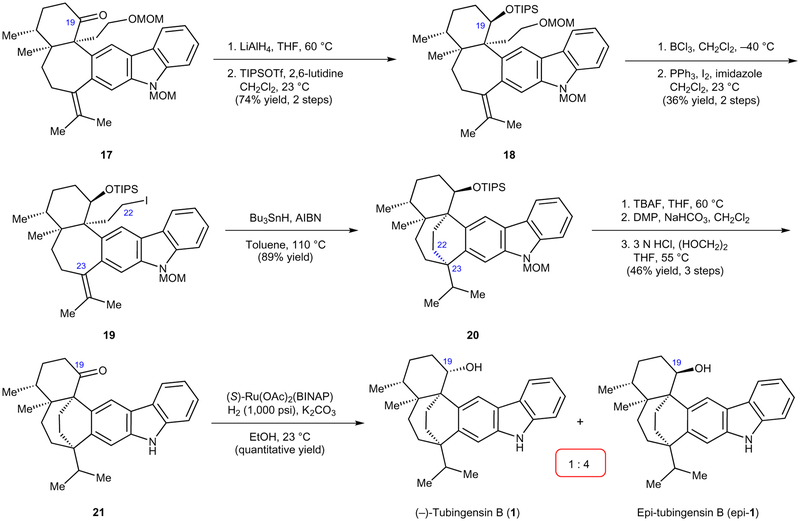
Figure 5 |. Radical cyclization and first generation total synthesis of tubingensin B.
Radical cyclization precursor 19 was prepared using several functional group manipulations. In turn, the radical cyclization led to the efficient assembly of the [3.2.2]nonane core and provided bicycle 20. Following further manipulations, 1 was obtained, albeit with its C19 epimer forming predominantly in the final step. MOM, methoxymethyl; THF, tetrahydrofuran; TIPSOTf, triisopropylsilyl trifluoromethanesulfonate; AIBN, azobisisobutyronitrile; TBAF, tetrabutylammonium fluoride; DMP, Dess–Martin Periodinane; BINAP, 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl.