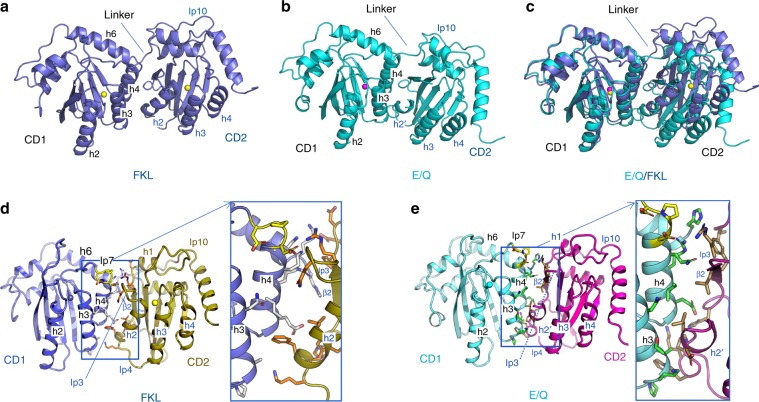
Fig. 1. Overall structural features of the double domain A3G from rhesus macaque (rA3G).
a, b Two different structures (FKL and E/Q) of the full-length rA3G (Supplementary Fig. 1 for 2nd structure assignment), showing that CD1 of both structures has a typical APOBEC fold with a Zn (sphere) at the active center. However, CD2 differs in the two structures. The CD2 of the FKL (A) has a canonical CD2 fold, but the CD2 of E/Q (B) re-folds helix 2 (h2) into a short 310 helix and a dramatically altered Zn-center conformation with no Zn-coordinated. c Overlap of the two full-length rA3G structures based on CD1, which reveals that the CD2 domains in the two structures have different orientations relative to their CD1s. d, e The CD1-CD2 interface interactions of the FKL (D) and E/Q (E) structures. Insets show residues (in sticks) directly participating in CD1-CD2 domain interactions in the two structures respectively (see Supplementary Fig. 4 for list of interacting interface residues in FKL and E/Q structures).