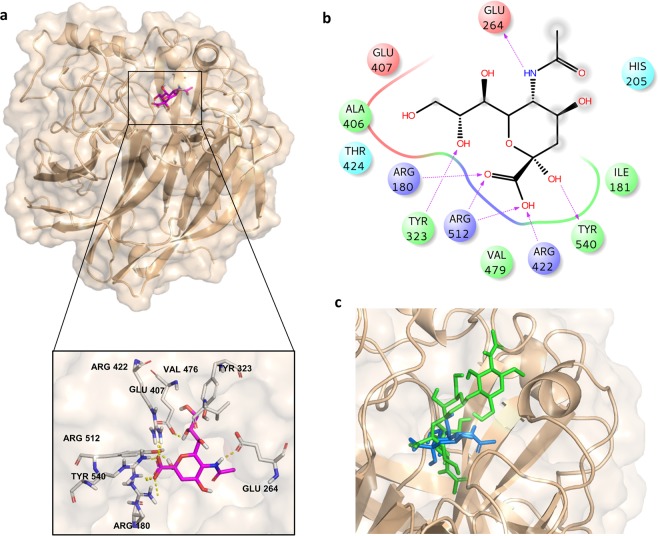
Figure 6.
3D model of MuV-HN in complex with β-Sia. (a) Close up view of β-sia binding mode at the MuV-HN active site, drawn by using Pymol 2.3 Software (https://pymol.org/2/). The main amino acid residues involved in the binding are represented in stick. (b) Two-dimensional plot illustrating the interactions of the β-Sia with the residues in the binding pocket of MuV-HN derived by a tool included in Maestro Version 10.5.014 (https://www.schrodinger.com/maestro). Dotted arrows represent hydrogen bonds with functional groups from side chains and solid arrows such with functional groups of the backbone. The residues shown, close to the ligand, are involved into hydrophobic and polar interactions. Although the different orientation inside MuV-HN binding site of the β-Sia with respect to the α-anomer in 1, the triad of arginine residues of the MuV-HN binding pocket was again engaged in strong ionic interactions with the Sia’s carboxylate. (c) Superimposition of β-Sia blue and trisaccharide 1 green in the MuV-HN binding site.