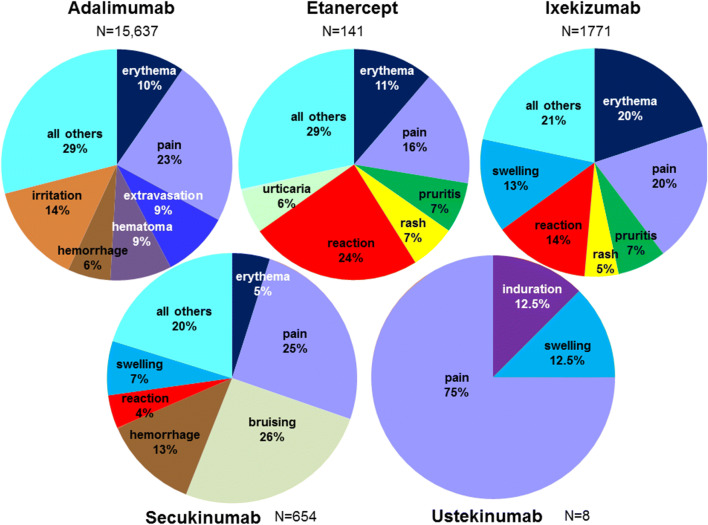
Fig. 1.
Proportion of injection site-related events in the Federal Adverse Event Reporting System (FAERS) database for each subcutaneous agent used to treat plaque psoriasis. For each drug (adalimumab, etanercept, ixekizumab, secukinumab, and ustekinumab), the most common types of injection site reaction-related events (preferred terms) are shown. Percentages were calculated based on the total number reports of injection site-reactions (N) for each drug