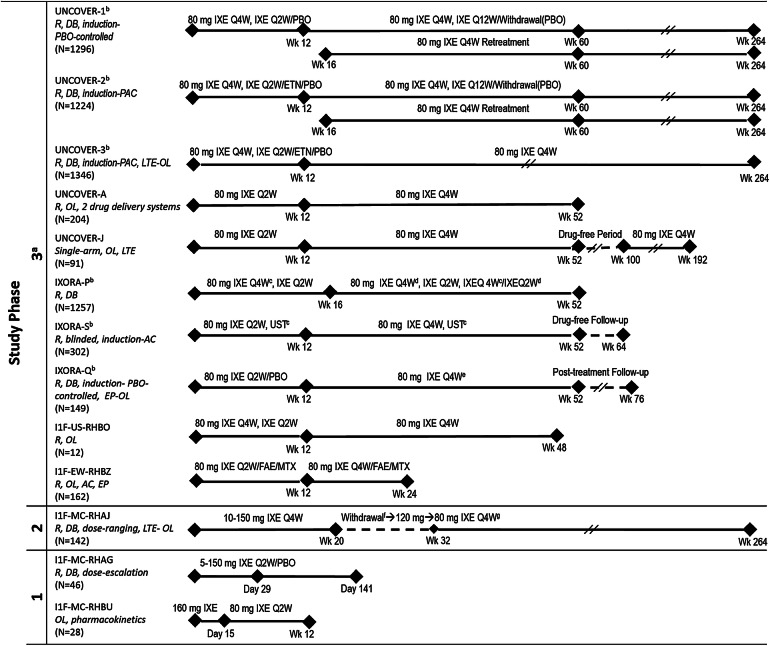
Fig. 1.
Designs and numbers of patients for all studies included in the analysis. aPatients receiving IXE received a 160-mg starting dose of IXE at week 0 prior to receiving 80 mg IXE (Q4W or Q2W). bGlobal studies. cPBO administered to maintain study blind. dStep-up criteria determined if dosing increased from 80 mg IXE Q4W to 80 mg IXE Q2W based on whether a patient achieved sPGA ≥ 2 at 2 consecutive visits during Wk 12 through Wk 40. eDosing increased from IXE Q4W to IXE Q2W based on investigator opinion from Wk 24 through Wk 40. fWithdrawal period (Wks 20–32); a patient was eligible for treatment with 80 mg IXE Q4W when improvement in PASI score from baseline was < 75% at any visit between Wks 20 and 32. gProtocol amendment-mandated dose regimen. Clinicaltrials.gov numbers: UNCOVER-1, -2, -3, -A and -J, NCT01474512, NCT01597245, NCT01646177, NCT01777191 and NCT01624233, respectively; IXORA-P, -S and -Q, NCT02513550, NCT02561806 and NCT02718898, respectively; RHBO, NCT02387801; RHBZ, NCT02634801; RHAJ, NCT01107457; RHBU, NCT02993471. AC active comparator, DB double-blind, EP optional extension period after Wk 24 during which patients received 80 mg IXE Q4W up to Wk 60, ETN 50 mg etanercept twice weekly, FAE fumaric acid esters 105-mg starting dose followed by 215 mg given orally 1–3 times per day, IXE ixekizumab, IXE Q2W ixekizumab every 2 weeks, IXE Q4W ixekizumab every 4 weeks, IXE Q12W ixekizumab every 12 weeks, LTE long-term extension, MTX methotrexate 7.5-mg starting dose up to 30 mg given orally once a week, N number of patients, OL open-label, PAC placebo-controlled and active comparator, PASI Psoriasis Area Severity Index, PBO placebo, R randomized, sPGA Static Physician’s Global Assessment, UST 45 mg ustekinumab given as subcutaneous injection for participants ≤ 100 kg and 90 mg subcutaneous injection for participants > 100 kg at weeks 0, 4, 16, 28 and 40, Wk week