Abstract
Magnetic Resonance Imaging (MRI) in paediatric cohorts is often complicated by reluctance to enter the scanner and head motion-related imaging artefacts. The process is particularly challenging for children with neurodevelopmental disorders where coping with novel task demands in an unfamiliar setting may be more difficult due to symptom-related deficits or distress. These issues often give rise to excessive head motion that can significantly reduce the quality of images acquired, or render data unusable. Here we report an individualised MRI training procedure that enables children with Autism Spectrum Disorders (ASD) to better tolerate the MRI scanner environment based on a child-focused approach and individualised familiarisation strategies, including a pre-visit interview, familiarisation package, and personalised rewards. A medical imaging mobile application was utilised to familiarise participants to multi-sensory aspects of the neuroimaging experience through a variety of themed mini-games and activities. The MRI training procedure was implemented for monozygotic twins (n = 12; 6 twin pairs; age range 7.1–12.9 years) concordant or discordant for ASD. MRI image quality indices were better or comparable to images acquired from a large independent multi-centre ASD cohort. Present findings are promising and suggest that child-focused strategies could improve the quality of paediatric neuroimaging in clinical populations.
Keywords: Neuroimaging, Magnetic resonance imaging, Paediatric, Neruodevelopmental disorders, Autism spectrum disorders
1. Introduction
Magnetic Resonance Imaging (MRI) is a non-invasive imaging technique for the study of brain structure and function, and has been widely used in both clinical and research settings to understand atypical brain development in neurodevelopmental disorders. A major challenge in neuroimaging studies is the problem of in-scanner head motion that can result in significant systematic effects on MRI image acquisition and analyses. In addition to motion-induced artefacts that distort structural and functional imaging, in-scanner head motion can introduce spurious variance that resemble anatomical or connectivity variation across individuals or between groups (Blumenthal et al., 2002; Power et al., 2015). The severity and frequency of motion-related artefacts are typically increased in patient groups, with higher in-scanner head motion compared to controls (Van Dijk et al., 2012). Observed differences on neuroimaging measures between clinical and control samples could thus be significantly confounded by systematic differences in head motion in quantitative analyses at the group level.
Motion-related issues are further exacerbated in paediatric cohorts with a higher tendency for increased movement in the scanner (Makowski et al., 2019). The MRI scanner environment is highly unusual and may be distressing for younger cohorts. Being in the MRI scanner can be a challenging and intimidating environment for any child, even more so for young individuals with neurodevelopmental conditions and associated comorbid deficits. During the scanning procedure, individuals are required to remain still for an extended period of time in a foreign, confined and noisy space in isolation. Children are more likely to be restless and reluctant to enter the scanner with reduced compliance, and the resulting discomfort or distress often leads to unwanted head or body movement that can severely impact the quality of imaging data acquired (Raschle et al., 2012). Indeed, success rates of paediatric functional MRI (fMRI) were lower in clinical groups across different populations in epilepsy (80%), attention-deficit/hyperactivity disorder (ADHD; 77%–81%), and Autism Spectrum Disorders (ASD; 70%), compared to that of neurotypical controls (87%; Yerys et al., 2009). Notably, 40%–50% of children from any clinical diagnostic group failed at least one scan. The primary contributing factor for failure was excessive head motion, as well as other issues including refusal to begin or complete a scan session, inattention, or refusal to enter the scanner. Older children and adolescents were more likely to undergo a successful scan compared to younger children between the ages of 4 and 9 (Byars et al., 2002; Yerys et al., 2009). While methods have been developed to retroactively adjust for motion artefacts or motion-distorted data, such strategies are generally not able to fully correct for artefacts and often result in significant loss of data, in particular for subjects with excessive in-scanner head motion. Reducing the likelihood of head motion and improving the overall scanner experience for the child in the outset before and during image acquisition could thus be a more effective approach to improve the the quality of images (Greene et al., 2018).
Given the significant difficulties in neuroimaging younger individuals, there have been increasing efforts to mitigate excessive head motion in paediatric clinical populations using various strategies before and during image acquisition without sedation (e.g. de Bie et al., 2010; Greene et al., 2016; Nordahl et al., 2016; Raschle et al., 2012). One approach has been to take children through a practice or mock MRI session before the actual scan, which has been shown to be an effective intervention for improving the success rate of scanning and data quality (Carter et al., 2010). However, research protocols commonly include full-battery neurocognitive assessments in addition to neuroimaging, and a typical study visit duration could take half a day or more for each participant. Together with unavoidable constraints on cost and time, there may be an increased tendency to prioritise data collection over pre-scan participant training and preparation for MRI in paediatric neuroimaging cohort studies. There have also been limited reports on the impact of fatigue or distress (symptom-related or otherwise) on scan performance in young children undergoing extensive testing procedures. At present, there remains a need to further investigate individualised strategies tailored to the specific needs of each child, and to target image acquisition challenges that could be unique across different neurodevelopmental disorders with distinct clinical profiles, or across different individuals within heterogeneous disorders that vary in symptom presentation.
Neurodevelopmental disorders are complex and often highly heterogeneous in aetiology, clinical features and outcome. The broad classification encompasses any condition associated with abnormal or disrupted brain development, including ASD, ADHD, genetic syndromes, congenital abnormalities, or epilepsy. Clinical presentation can involve a wide range of neurocognitive and psychiatric symptoms that often overlap across conditions beyond nosological boundaries (Thapar et al., 2017). For example, in addition to the core symptom features of hyperactive-impulsiveness or inattention in ADHD, patients often present with cognitive deficits, emotional and behavioural dysregulation, or with co-occurring anxiety, depression or oppositional defiant disorder (Shaw et al., 2014; Wåhlstedt et al., 2009). Such co-morbid deficits are often masked by primary symptoms and remain underdiagnosed, and may be even more difficult to detect if they occur at a sub-clinical level. Similarly, there is significant heterogeneity in the phenotypic expression of ASD in symptom presentation and severity, and across adaptive functioning, and cognitive and language abilities (Geschwind and Levitt, 2007). Common comorbidities in ASD include ADHD or anxiety, as well as associated communication problems and intellectual disability (Simonoff et al., 2008). ASD is also associated with idiosyncratic symptom features in communication deficits, sensory hypersensitivity, or resistance to change or novel stimuli, and clinical profiles could vary significantly between individuals with the condition (Johnson and Myers, 2007). Difficulties in paediatric image acquisition are often compounded in heterogeneous neurodevelopmental conditions, with complex and highly varied presentation across individuals that contribute to increased risk of in-scanner motion, as well as the distinct lack of subject-specific methods or strategies to mitigate such issues. The increased demand of coping with novel task-demands or significant distress in foreign environments often makes image acquisition a challenge in such populations with heterogeneous clinical profiles (Hallowell et al., 2008; Pua et al., 2017).
Reliable image acquisition of brain structure and function on MRI has thus been a longstanding challenge in paediatric cohorts with neurodevelopmental conditions, with motion-related imaging confounds as a major contributing factor. While brief or limited MRI preparation may be sufficient for neuroimaging neurotypical children, individualised strategies and disorder-specific MRI familiarisation procedures are likely to be more effective for image acquisition in paediatric clinical populations. Building on current knowledge and previous recommendations, we designed a training protocol based on an individualised child-focused approach to prepare children for MRI neuroimaging. The primary goal was to enable participants with ASD to better tolerate MRI imaging with acceptable levels of head motion. Here we report the training protocol and quality of MRI image acquisition implemented for a locally recruited monozygotic twin sample concordant or discordant for ASD. Mean framewise displacement (FD) as an estimate of change in head motion across image volumes that strongly relates to motion artefacts, and rate of change of acquired signal across the whole brain at each frame (DVARS; Power et al., 2012; Smyser et al., 2010), were used as data quality indexes to compare image quality of the locally recruited sample to a large multi-centre ASD cohort.
2. Methods
2.1. Participants
Participants (n = 12) were locally recruited from Twins Research Australia (TRA) and an ongoing epigenetics study on ASD at The Royal Children’s Hospital (RCH; HREC 33208C). Inclusion criteria were monozygotic twins concordant or discordant for ASD between the ages of 5–18 years, of either sex, and raised in the same household in the greater metropolitan area of Melbourne, Victoria. The lower limit of the age range was selected due to the known challenges of scanning tolerance and motion artefacts in imaging young children, especially in atypical neurodevelopmental populations. ASD diagnosis was previously determined by clinical assessment on the gold standard Autism Diagnostic Observation Schedule-2 (ADOS-2) or by the TRA with supporting medical documentation of prior diagnosis and assessment. Zygosity status of twin pairs was confirmed with genetic testing using a twelve-marker panel following DNA extraction from buccal swabs. Results of zygosity testing were only released to parents upon request to respect the privacy of families. Informed consent was obtained from all participants and the research study protocol was approved by the RCH Human Research and Ethics Committee (HREC 36124C). All research was performed in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Table 1 provides a descriptive summary of participant demographics.
Table 1.
Participant demographics and data quality indexes.
| Twin Pair | Gender | Age (years) | ASD Diagnosis | SRS | FD (Run 1) | DVARS (Run 1) | FD (Run 2) | DVARS (Run 2) | Invalid Volumes (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 10.43 | Yes | 51 | 0.20 | 1.16 | 0.40 | 1.14 | 15.4 |
| 1 | Male | 10.43 | Yes | 101 | 0.13 | 1.20 | 0.25 | 1.19 | 5.40 |
| 2 | Male | 9.82 | Yes | 116 | 0.87 | 1.14 | 0.48 | 1.16 | 43.2 |
| 2 | Male | 9.82 | Yes | 136 | 0.31 | 1.17 | 0.35 | 1.25 | 13.6 |
| 3 | Female | 7.1 | Yes | 145 | 0.16 | 1.27 | 0.15 | 1.35 | 0.40 |
| 3 | Female | 7.1 | Yes | 124 | 0.13 | 1.27 | 0.13 | 1.26 | 2.00 |
| 4 | Male | 12.85 | Yes | 139 | 0.14 | 1.16 | 0.18 | 1.19 | 4.20 |
| 4 | Male | 12.85 | No | 109 | 0.30 | 1.18 | 0.30 | 1.12 | 11.2 |
| 5 | Male | 12.17 | Yes | 122 | 0.19 | 1.20 | 0.22 | 1.21 | 8.80 |
| 5 | Male | 12.17 | No | 49 | 0.16 | 1.15 | 0.15 | 1.18 | 3.60 |
| 6 | Female | 7.49 | Yes | 100 | 0.19 | 1.16 | 0.18 | 1.12 | 3.60 |
| 6 | Female | 7.49 | No | 7 | 0.21 | 1.22 | 0.22 | 1.20 | 9.80 |
Note: SRS: Social Responsiveness Scale-2 total score; FD: Mean framewise displacement; DVARS: standardised DVARS measure (rate of change of image intensity relative to the previous timepoint); Invalid volumes: Percentage of outlier volumes identified for scrubbing/censoring based on thresholds for scan-to-scan changes in subject-motion parameters (0.5 mm) and global signal (Z = 3) using the ARtifact Detection Tools (ART) outlier detection procedure (Fischer et al., 2014).
2.2. Pre-visit preparation
Standard MRI visit protocols were modified for each participant to accommodate expected difficulties specific to ASD. Key goals were to familiarise participants to the MRI scanner environment, and to improve tolerance to the loud and repetitive acoustic noise in the scanner. First, parents of children that met the eligibility criteria were contacted for a pre-visit interview one to two weeks before the MRI visit. A brief semi-structured clinical interview was conducted by a board-certified provisional psychologist, with the aim of gathering relevant information to develop reinforcement and motivation strategies individually tailored to each child. The interview included queries about general interests and hobbies, and reward strategies that were effective for each child. The goal of such strategies was to increase the likelihood of desirable behaviours with positive reinforcement in the form of verbal or material rewards. The approach has previously shown to be effective for behavioural modification outcomes in ASD (Gena et al., 2005). Triggers that typically preceded idiosyncratic episodes of distress or repetitive symptoms were also identified for children with an ASD diagnosis. Potential strategies for intervention were explored to better understand the parent-child interaction during such episodes, and possible approaches to manage episodes that may occur on-site during the MRI visit.
Parents were provided with an in-house MRI familiarisation package that comprised of three main components:
-
1)
A brief, two to three minutes duration, MRI orientation video1 filmed at the RCH introduces the child to locations in the hospital that would be encountered during the actual on-site visit, such as the patient waiting area, preparation room, and MRI scanner.
-
2)
An ‘Okee in Medical Imaging’ mobile application developed in collaboration by RCH Medical Imaging, Educational Play Therapy, and Educational Resource Centre and a digital agency2. This free-of-charge application can be easily accessed on most smart device platforms, and was specifically designed to familiarise young children with aspects of neuroimaging. The application contains a suite of 9 interactive games and activities with an undersea adventure theme (see Supplementary Appendix A1), and also provides useful information and tips for parents to help their child prepare for the visit.
Training games. These activities were designed to introduce and illustrate key aspects of an MRI scan experience. The ‘Keeping Still’ activity teaches children to keep still when instructed, only moving when appropriate. The game requires the child to maintain the position of a jellyfish character when a shark appears on the screen. The novel use of gyroscope sensors available in most smart devices allows immediate visual and audio feedback on the degree of motion during the activity through the application (see Supplementary Appendix A2). This provides a unique opportunity for the child to learn when movement was appropriate or not as instructed, and for parents to guide the child and to observe adherence to specific instructions. The aim of these games was to enable each child to appreciate the concept of minimising in-scanner movement across different intervals, and to remain still when instructed. The ‘Breathing’ task allows children to practice holding and regulating their breath using visual feedback from an animated puffer fish, and the ‘Contrast’ task introduces the concept of contrast injections for contrast MRI through a game that requires the child to fill a cartoon squid with ink.
Adventure games. Other games introduced and familiarised the child to different components of an MRI scanner through a themed undersea submarine pretend-play simulation. In one interactive activity, the child was tasked to build and paint their own ‘MRI submarine’ (see Supplementary Appendix A3). The goal of this activity was to gradually familiarise the child to how an MRI machine might look and sound like throughout the game. The child was introduced to the main features of the scanner, including the bore and participant bed, followed by different samples of MRI gradient noises that were framed as part of the submarine launch sequence. The Okee application also contains similar games designed for other medical imaging modalities such as CT, ultrasound, X-ray, and nuclear imaging.
3) Parents were provided with recorded audio clips of noise emitted by the MRI scanner that each child would later experience during the actual scan (see Supplementary material Appendix B). The acoustic noise is generated by vibrations of the MRI gradient coil, and the cyclic repetitive pattern or loud volume may lead to discomfort resulting in an undesirable increase in head or body motion (Cho et al., 1997; Counter et al., 1997). The audio clips in the familiarisation package comprised of the actual acoustic noise generated by the specific MRI sequence used in the research study. Samples were recorded in real-time from our scanner and extracted by imaging technicians. The site-specific audio sample was necessary because the generated acoustic noise could vary between scanner sites, equipment, and acquisition sequences. Parents were given instructions to familiarise their children to the MRI sound clips on a daily basis, beginning with a brief playback of the audio clip on the initial day followed by a gradual increase in the length of playback over a one-week period. Any type and level of distress elicited by the audio clips were to be noted. Parents were asked to introduce their children to the MRI orientation video at least once, and to allow them to explore the Okee medical imaging application. Parents were also encouraged to bring comfort objects and a favourite movie for their children to reduce distress during the visit. Links to resources and materials from the familiarisation package are provided in Supplementary materials.
2.3. Study visit
The on-site visit at the hospital for an MRI comprised of two key phases: a mock MRI training session and the actual MRI scan. A total of 1.5–2 hours was required per twin participant for the visit, and they were allowed to rest while their co-twin was being scanned. The overall visit included a one-hour long lunch break. Based on information gathered during the pre-visit interview, both the mock and MRI scan were personalised to suit individual needs and language ability of each participant. For example, if parents noted that their child tended to become distressed with unfamiliar people or situations, rapport building would be a key focus during the initial contact and period of interaction with families. Nonverbal approaches and materials were emphasised for participants with poorer language ability. Staff identification cards were also used to facilitate the learning of name-face associations based on explicit audio-visual cues, rather than a generic or brief introduction by name.
2.3.1. MRI orientation session
The MRI training phase during the visit involved an orientation session to explain the day’s schedule and the MRI process (30 min), followed by a mock MRI simulation session (30 min). The orientation session was conducted with parents or caregivers present, and both twins together. The visit began with an outline of the planned activities for the day. Given that an established and predictable schedule is often useful to facilitate the completion of tasks in individuals with ASD (Horner et al., 2002), the aspect of time was clearly emphasised, to the point of providing specific start and end times for each activity if needed. A visit map of the appointment schedule was prepared as a visual aid in the form of a flowchart with photographs of the actual location or item associated with a particular activity for each task (see Supplementary materials Appendix C):
-
1
Introduction (photograph: hospital common waiting area)
-
2
Visit orientation (photograph: mock interview room)
-
3
Mock training session (photographs: MRI mock scanner, DVD library of movies)
-
4
MRI waiting area (photograph: MRI reception desk)
-
5
MRI scanning session (photograph: MRI scanner)
Photographs of key locations or equipment in the flowchart were used as visual cues to familiarise the child with each segment of the visit. The MRI orientation video from the familiarisation package was presented again to each participant, and they were encouraged to explore the Okee mobile application. Participants were then informed about rewards they would receive at the end of the visit. These rewards were personalised for each individual based on motivation strategies discussed with parents during the pre-visit interview, and served as a form of positive reinforcement. The items were specific to each child, and ranged from art materials to trading cards or a soft toy. Each participant further received a certificate for completing the mock training, a certificate for completing the MRI scan, and screen captures of their scan as a memento.
Next, the child was engaged in a play-based session to learn about the MRI scanning process. A pictorial storyboard comprising of photos of on-site facilities and equipment was used to describe the an MRI scan in simple terms. The importance of keeping still was heavily emphasised, and pictures of scans with excessive motion artefacts were shown to highlight the effect of head motion on image quality. The acoustic noise experienced during the scan was described to be very similar to the audio clips the child would have been exposed to on a regular basis in the week prior to the visit. An illustrated storybook incorporating various elements of the MRI scan procedure in the form of a social story was available for the same purpose. After the storyboard presentation, the scanning process was recreated for the child with a pretend play-set with dolls and customised wooden blocks, where age-appropriate. The playset was used to sequentially explain what a participant might experience during a typical scan, and to introduce different components of the scanner such as the horizontal and vertical movement of the MRI bed and the helmet head coil.
2.3.2. Mock MRI simulation
In the subsequent mock MRI simulation phase, participants were given the opportunity to interact with a non-functional mock-up MRI scanner. The mock scanner included various MRI scanner components that the child would encounter during the scan, such as the moveable patient bed, head coil, and headphones. Participants were systematically introduced to the scanning process using a task-analysis approach, in which a task was segmented into a sequence of smaller steps or activities (Hernandez and Ikkanda, 2011; Nordahl et al., 2016). This allowed participants to familiarise themselves with the scanner environment at their own pace, and to reduce the risk of the child becoming overwhelmed. The stepwise approach offered the opportunity for additional instruction or support at each step if participants showed signs of distress or anxiety, and progression to the next stage only occurred if the previous step was comfortably completed by the child. Stages for the mock scanning process, adapted from the task analysis-based approach proposed by Nordahl et al. (2016), were as follows:
-
1
Entering the mock scanner room
-
2
Exploring the mock scanner (front and rear)
-
3
Playing a selected DVD movie (from home or available library)
-
4
Approaching the mock scanner bed
-
5
Operating the mock scanner bed (vertical and horizontal movement controls)
-
6
Sitting on the mock scanner bed
-
7
Putting on headphones
-
8
Lying on the bed
-
9
Tolerating vertical movement of mock scanner bed
-
10
Placement of the head coil
-
11
Listening to MRI gradient noise outside the scanner (external playback of recorded MRI audio clips from familiarisation package)
-
12
Lying still on mock scanner bed for one minute
-
13
Tolerating horizontal movement of mock scanner bed into scanner
-
14
Listening to the MRI gradient noise in scanner
-
15
Increasing amount of time to lie still (∼5 min) while listening to MRI audio clip in the scanner, and watching a movie
The stepwise approach was used to gradually familiarise each participant with the scanning process at their own pace and comfort levels. Verbal encouragement or material rewards were used to reinforce successful completion of each step where necessary. If participants brought along a comfort object, they were allowed to hold on to it when entering the mock scanner. If the object was an action figure or soft toy, it was used as a mock participant to demonstrate different steps of the scanning process. Participants were also allowed to observe their co-twin undergo the mock simulation process to facilitate learning through peer-modelling and sibling involvement (Shivers and Plavnick, 2014). Participants took turns observing their co-twin sibling for the full duration of the mock training session, before undergoing the training themselves while their co-twin observed.
To estimate participant head motion in the mock scanner, measurements from an accelerometer device were recorded during the final step, where each child was instructed to remain still for five minutes on the scanner bed while listening to a playback of MRI gradient noise, and watching a movie. The accelerometer device (3-axis, 50 Hz sample rate, 15-bit resolution) was attached to the mock scanner headphones and connected to a computer terminal in the same room, providing real-time feedback of participant motion in the mock scanner simulation. Using a real-time display of signal captured from the accelerometer device, participants were given visual feedback to understand how excessive head motion could introduce noise and visible fluctuations in the signal being recorded. The goal of the mock scanner simulation was to facilitate successful and comfortable completion of a simulated MRI scan with minimal movement, and the suitability of each participant for an MRI scan was assessed at the end of the training session. Participants and their parents were then debriefed together to explore and address any further concerns. Families were allowed to take pictures with the mock scanner, and each child was rewarded with a certificate of completion for the mock training phase. Participants were allowed a one to two hour break before their MRI scan.
2.3.3. MRI image acquisition
Multimodal MRI data was acquired on a 3-Tesla Siemens Tim Trio MRI scanner (Siemens, Erlangen, Germany) with a 32-channel head coil. A modified multi-echo magnetization prepared rapid gradient-echo (MEMPRAGE) sequence was used to acquire T1-weighted anatomical images (TR = 2530 ms, TE = 1.77, 3.51, 5.32, 7.20 ms, TI = 1260 ms, flip angle = 7.0 deg, voxel size = 0.9 × 0.9 × 0.9 mm, FOV read = 230 mm). Navigator based prospective motion correction was implemented with Siemens in-scanner motion correction (MoCo), where field-of-view and slice positioning were updated to adjust for motion in real-time to reduce motion artefacts and improve image quality. Participants were allowed to watch a movie of their choice during image acquisition. Task-free blood oxygen level-dependent (BOLD) fMRI data to estimate functional connectivity between brain regions based on intrinsic correlated neural activity at rest was acquired with multi-band accelerated EPI sequences across two separate runs within the same scanning session (TR = 1500 ms, TE = 33 ms, volumes = 250 voxel size = 2.5 × 2.5 × 2.5 mm, multi-band factor = 3). Participants were instructed to keep their eyes open and to focus on a fixation cross during the scan.
The overall scan duration for each subject was around 45 min. Participants were not permitted to move during image acquisition, but were allowed brief periods of rest between sequences. All participants successfully completed the scans without withdrawing or displaying significant signs of distress. Each child was rewarded with another certificate of completion, along with their individual rewards as described above.
2.4. Data quality analyses
Mean framewise displacement (FD) and DVARS were used as quality control metrics from the MRI Quality Control Tool (MRIQC; Esteban et al., 2017). The tool integrates modular sub-workflows dependent on common neuroimaging software toolboxes. The data was first minimally preprocessed in the MRIQC anatomical workflow with skull-stripping, head mask and air mask calculation, spatial normalisation to MNI space, and brain tissue segmentation. T1-weighted images were visually inspected for ringing artefacts, blurred grey- and white-matter boundaries, and background noise (Pardoe et al., 2016). Head motion correction was performed in the functional workflow with AFNI 3dvolreg (Cox, 1996). The algorithm computes head realignment across frames, registering each image to a base reference volume using a six-parameter, rigid-body transformation (angular rotation and translation). Framewise displacement (FD) is a data quality index expressing instantaneous head-motion based on change in head position across frames. FD is the estimated spatial deviation between the reference volume and all other volumes derived from the sum of the absolute values of the differentiated rigid-body realignment estimates. Rotational displacements were computed as the displacement on the surface of radius 50 mm. Framewise displacement was additionally estimated with a different tool (FSL mcflirt) for validation (Jenkinson et al., 2002). DVARS is another quality index that estimates the rate of change of BOLD signal across the whole brain at each frame (Smyser et al., 2010; Power et al., 2012). The change in image intensity compared to the previous timepoint was computed by differentiating the volumetric timeseries and obtaining the root-mean-square signal change. The metric was normalised with the standard deviation of the temporal difference timeseries to allow comparisons between different imaging sites and scanners (Nichols, 2017).
For multi-centre and multi-cohort comparisons, data quality metrics were obtained from the Autism Brain Imaging Data Exchange-II (ABIDE-II; Di Martino et al., 2017). ABIDE-II is a publicly available aggregation datasets from n = 487 individuals with ASD and n = 557 controls (age range: 5–64 years). Site-specific protocols for participant preparation and image acquisition are available.3 Quality metrics of ABIDE-II data were previously derived using the Quality Assessment Protocol (QAP) from the Preprocessed Connectomes Project (Shehzad et al., 2015).
3. Results
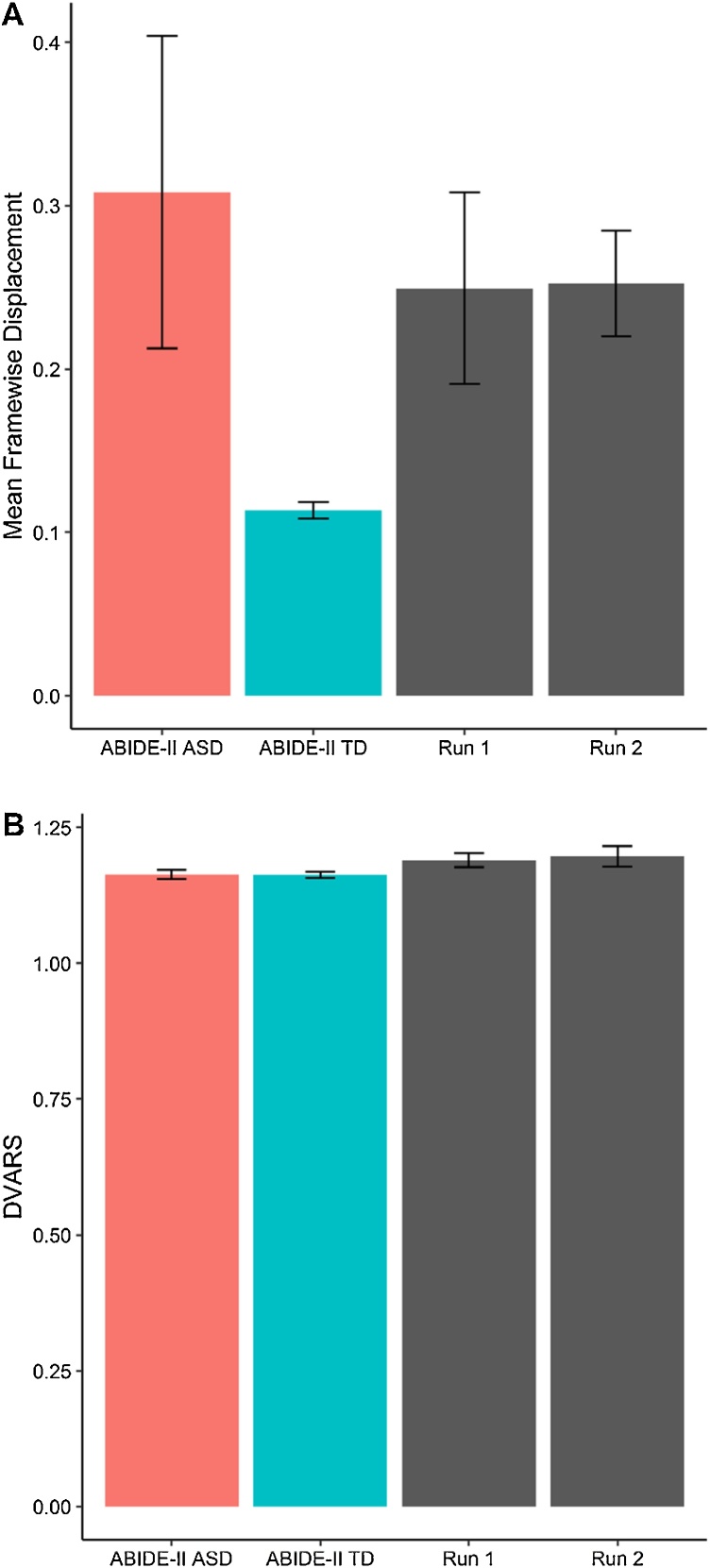
All participants completed each stage of the MRI training protocol (pre-visit phase, orientation session, mock MRI simulation and evaluation). Parents provided verbal confirmation of participant engagement with the familiarisation package. Materials were also presented to participants again during the orientation session. The entire duration of the hospital visit ranged from four to five hours per family, including a one-hour lunch break. Mean FD across the locally recruited sample representing instantaneous head motion for both task-free sequences (Run 1: M=0.25 mm, SD=0.20; Run 2: M=0.25 mm, SD=0.11) were more favourable compared to individuals with ASD from the ABIDE-II cohort (M=0.31 mm, SD=2.37), but not typical controls (M=0.11 mm, SD=0.14). FD results obtained from FSL mcflirt (Run 1: M=0.23 mm, SD=0.19; Run 2: M=0.23 mm, SD=0.10) were comparable to the output from AFNI 3Dvolreg, suggesting stability of FD estimates independent of analysis method. DVARS as the standardized root mean squared change in BOLD signal intensity from one volume to the next in the local sample for both runs (Run 1: 1.19; Run 2: 1.20) were similar to individuals from the ABIDE-II cohort (ASD: 1.16; controls: 1.16). Only one participant from the local cohort failed to meet the threshold of <0.5 mm for acceptable FD due to excessive head motion (95.8% success rate).The same participant however demonstrated improved FD (0.48 mm) below the threshold in the repeat run later acquired within the same scanning session, a significant reduction from the observed FD in the initial run (0.87 mm).
Based on monozygotic twin regression modelling from Carlin et al. (2005), within-twin-pair differences in outcome variables were regressed onto within-pair differences in independent variables. As expected, FD was strongly related to DVARS (Run 1: t = 3.79, R2=0.69, p=0.01) suggesting a relationship between head motion and BOLD signal intensity change between volumes. The association was present but less robust in the repeat acquisition (Run 2: t = 2.24, R2=0.40, p = 0.07). Intrapair associations between FD from the initial and repeat task-free acquisition were non-significant (t=2.386, R2=0.44, p=0.06). Vertical head motion recorded during the mock training simulation predicted in-scanner head motion during the first task-free acquisition (Run 1: t=-2.52, R2=0.47, p=0.05; Run 2: t=-0.81, p=0.45; Z-axis root-mean-squared-successive-difference). There were no within-twin-pair associations for FD and DVARS in the initial (FD: r=0.57, p = 0.24; DVARS: (r=0.34, p = 0.51) and repeat acquisition (FD: r=0.57, p = 0.24; DVARS: r=-0.56, p = 0.25).
4. Discussion
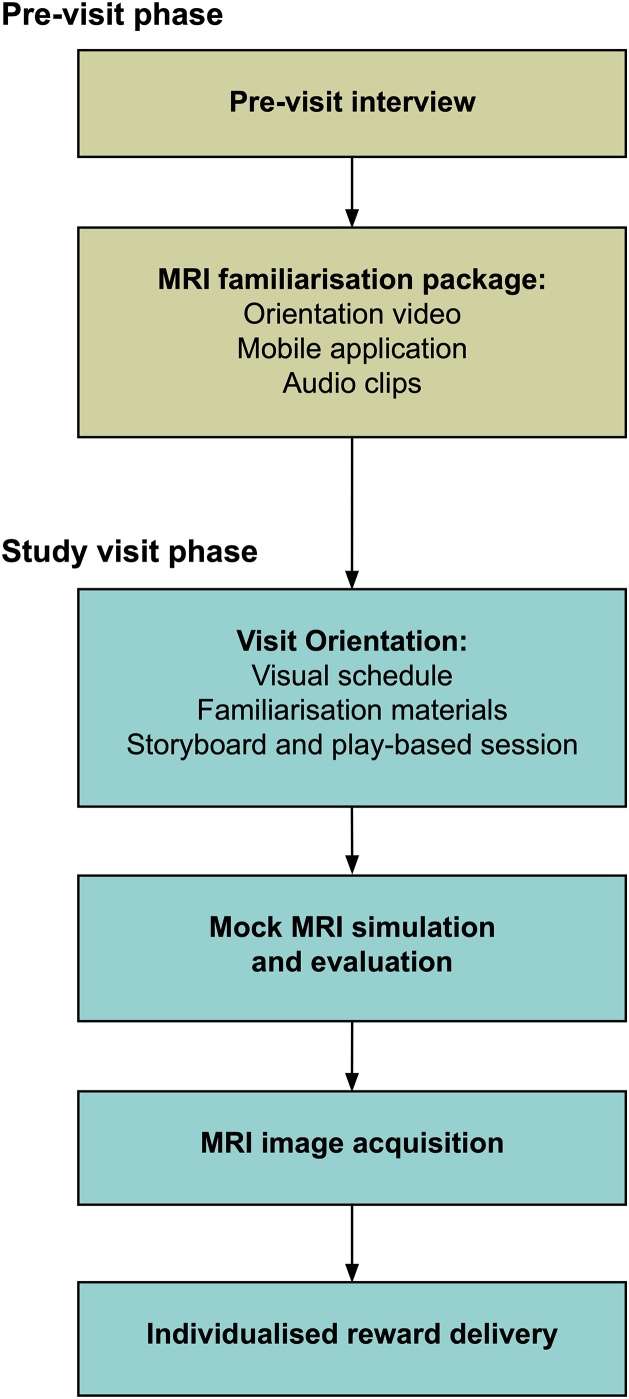
This paper presents an MRI training procedure for paediatric cohorts implemented in twin participants with or without ASD. Key components were a personalised child-centred approach and an MRI familiarisation strategy. Briefly, a child-focused strategy was developed for each individual participant based on a pre-visit clinical interview with parents to identify effective methods for motivation and potential distress or symptom triggers. Families participated in an MRI familiarisation procedure one week prior to the visit, during which participants were gradually introduced to recordings of site-specific MRI acoustic gradient noise on a daily basis. Parents were also provided with materials such as an MRI orientation video and games application to familiarise their children with the MRI scanner, and were encouraged to engage in these activities together. On the actual day of the visit, a significant proportion of time was allocated for the MRI training protocol. Key components were an orientation phase with the use of visual aids and previously distributed familiarisation materials, a play-based session incorporating an MRI playset, and a task-based approach was used to simulate the scan procedure in a mock MRI scanner before the actual scan. Implementation of the process was flexible and readily adapted based on information gathered from the pre-visit interview to best suit the needs and ability of each participant (see Methods for full details; Fig. 1).
Fig. 1.
Flowchart of a child-focused paediatric MRI training protocol. A pre-visit interview with parents was used to develop an individualised strategy for MRI training, together with an MRI familiarisation procedure using a mobile application and site-specific materials. During the visit, participants went through a detailed orientation session that introduced key aspects of the MRI training and the scanning process, followed by a mock MRI simulation prior to the actual MRI scan. Participants were rewarded with personalised incentives.
Based on the thresholds for acceptable levels of head motion and image artefact quality control, only one participant failed to meet criteria (95.8% success rate). Mean head motion was significantly reduced in repeat image acquisition within the same session for the same participant. Across all participants, head motion in the initial acquisition was not significantly associated with that observed in the later run. Overall, this suggests that repeat acquisition sequences may be an effective tempering strategy for individuals with excessive head motion. The utility of repeating sequences of interest is in agreement with previous recommendations to increase power and volumes retained after motion correction and denoising procedures in similar paediatric cohorts (Greene et al., 2016). Importantly, image quality indexes for head motion and signal change in the present study exceeded or were similar to the quality of data from the multi-centre ABIDE-II cohort for individuals with ASD (Fig. 2). As expected, individuals with ASD across all cohorts also displayed higher head motion compared to neuroptypical controls. Given the difficulties of neuroimaging paediatric cohorts, data quality outcomes based on these findings suggest that the MRI training procedure may be useful in mitigating motion-related artefacts.
Fig. 2.
Image data quality indexes with comparisons to the ABIDE-II cohort. Mean framewise displacement: Higher values indicate increased volume-to-volume head motion; DVARS: Standardized DVARS measure. Higher values indicate increased change in image intensity across volumes; ABIDE-II ASD: ABIDE-II individuals with ASD; ABIDE-II TD: ABIDE-II neurotypical controls; Run 1: Initial task-free sequence for local sample; Run 2: Repeated task-free sequence for local sample.
4.1. A child-focused approach
The common adage that no two individuals on the ASD spectrum are alike encapsulates the understanding the expression of core symptoms in each child with ASD is likely to be idiosyncratic due to the complex phenotypic heterogeneity of the condition (Masi et al., 2017). The benefits of child-focused interventions in ASD based on individualised techniques to target specific behavioural outcomes or symptom reductions are well established (Tonge et al., 2014). Extending the efficacy of this approach to MRI training, the protocol implemented here was designed to be personalised and tailored to the individual participant. Information gathered in the pre-visit interview critically informed strategies for the mock MRI training session during the visit, where different components of the protocol were emphasised or adapted based on the child’s needs or level of functioning.
The pre-visit interview facilitated information gathering that was critical to the planning and delivery of the training protocol for each participant. Functional assessment was previously reported to be the most consistent factor in predicting intervention success, where the effectiveness of intervention appeared to increase with the precision of assessment in some form of interview, direct observation or functional analysis (Horner et al., 2002). In particular, informant information was key to providing a comprehensive picture of behaviours and symptoms of the individual child across multiple environments and settings (Stratis and Lecavalier, 2014). In the context of the present study, parent information derived from the pre-visit interview facilitated the development of a personalised strategy for each child during the mock MRI training phase. Individualised information about each child critically supports the later training phase that incorporates several elements of Positive Behaviour Support (PBS) interventions, such as removing triggers or antecedent events preceding undesirable behaviours, teaching new skills, and rewarding positive behaviours (Carr et al., 2002). PBS outcomes have been shown to further improve when informant information across different contexts was available, in addition to partnership efforts with multiple parties including the family and the school (Harvey et al., 2003). For example, parents of a participant with ASD from one family in our study reported sensitivity to loud noises that could trigger a behavioural episode. This information was used to modify the training protocol for this particular participant, such that noise modulation and gradual introduction of the MRI gradient noises was of specific focus during the initial interview with the child and mock preparation phase. Another family reported that their child with ASD required rigid routines and expectations about the start and end times of different activities. For this particular case, a larger emphasis was placed on the visual schedule map and timelines to inform the child on the expected schedule for each activity of the day. Together, these individualised strategies based on informant reports gathered prior to the MRI visit were observed to be effective in avoiding or reducing discomfort in children during the research study.
Personalised and effective positive reinforcement strategies were developed together with parents in a collaborative partnership to better appreciate the interests, motivations, and fears of each child during the pre-visit interview. Using this approach, we found that participants were more likely to comply with instructions and better tolerate the MRI preparation and scanning procedures. Given that social or verbal praise and attention may not be as rewarding for some children with ASD, the identification and implementation of individually functional reinforcers was a critical component of this strategy. Personalised rewards tailored to the interests of each child (e.g. favourite snack or toy) could serve as more effective positive reinforcers (Horner et al., 2002). In the present study, rewards were carefully selected based on a discussion with parents prior to the visit. Individual rewards ranged widely from sports trading cards, nail painting kits, food vouchers for the child’s favourite outlet, animated character stickers (e.g. Spider-man, Barbie, Star Wars), in addition to each child’s completion certificates in their name. During the visit, participants were informed of their individual rewards in the initial interview, and most demonstrated high levels of motivation to comply with instructions. Participants were also reminded of the rewards they would receive at the end of the session during periods of restlessness or discomfort, or when they found it challenging to remain still. In contrast, generic souvenirs or cash vouchers as common tokens of appreciation for research participation are likely to be much less attractive to children, and would have been less effective as positive reinforcers. As these rewards were highly specific to the interests of each child, the reward for one participant may not be equally attractive to another individual, highlighting the importance of personalised reinforcers. Essentially, child-focused approaches to meet task-demands were based on adaptable strategies to uniquely support the needs of each individual child and family (Trivette et al., 2000). Throughout the entire process, parents and participants were also reassured that the child’s well-being took priority over research outcomes, and were allowed to withdraw at any stage during the training or scanning phase of the visit.
4.2. Familiarisation strategy
Another key aspect of the training protocol was the incorporation of familiarisation strategies for the MRI scanner environment, specifically targeting participant tolerance to the loud and repetitive acoustic noise in the scanner. Drawing from basic elements of graduated exposure therapy (Craske et al., 2014; Wolpe, 1968), participants were systematically exposed to variations of the scanner environment that progressively became closer approximations to the actual scan. Graduated exposure techniques have been shown to be effective interventions to overcome setting avoidance or reduce anxiety in high- and low-functioning individuals with ASD (Hagopian and Jennett, 2008). Similar to our observations, the combination of graduated exposure and positive reinforcement to reduce setting and activity avoidance was particularly effective (Schmidt et al., 2013). Initial exposure to the scanner environment began with the audio playback of the acoustic noise in their homes one week prior to the hospital visit, followed by the site-specific orientation video and activities from the mobile application that visually introduced the actual scanner location in the hospital. This prepared each participant for the on-site orientation to the scanner environment during the visit, and the graduated mock scanner simulation leading up to the actual MRI scan. Progression was based on successful completion of the previous stage with minimal anxiety or discomfort, and the speed of progression was adjusted depending on the performance of each participant where applicable. The goal was to facilitate desensitisation through gradual exposure to the target stimulus, allowing participants to develop tolerance or habituate to in-scanner noise over time and to minimise anxiety-related or avoidant responses. A flowchart of on-site photographs mapping the schedule and length of activities was additionally used to visually structure the visit for participants. The implementation and utility of a visual schedule is similar to the Picture Exchange Communication System (PECS) in which instruction or learning is supported by visual elements that complements or minimises verbal input. The strategy offers the child a consistent and predictable system to understand a sequence of activities or tasks, and has been highly effective in facilitating communication and instruction in this population (Schneider and Goldstein, 2010; Shane, 2006).
Another important feature of the familiarisation procedure was high family-involvement. The delivery of the MRI familiarisation materials by a parent in the home environment is supported by previous reports showing that efficacy of ASD interventions improved when the method of delivery included familiar agents (e.g. parents or teachers) in typical contexts (e.g. home or school; Horner et al., 2002). As children with ASD may have difficulty applying learned skills or outcomes in a novel settings, interventions or learning strategies should be initiated by a familiar person and integrated with a child’s daily routine and activities in a natural learning environment (Childress, 2004). This was an important lead-up to the mock MRI on the actual day of visit, where parents were encouraged to participate and interact with their children throughout the session using the same materials. The clinician or researcher facilitating the training would build on concepts or MRI-related stimuli previously introduced by parents in their home, and the bridged experience was less likely to be overly novel or intimidating for the child. Given that each child received mock training together with their co-twin, each participant also had the opportunity to observe their sibling as a natural peer model. Systematic review findings suggest that sibling-involvement in ASD interventions were similar to peer-mediated strategies, with positive outcomes in increased skill acquisition or reductions in unwanted behaviours (Shivers and Plavnick, 2014). Consistent with our protocol design, the effectiveness of sibling peer-modelling was further complemented by peer and parental prompting, directed instructions, and positive reinforcement strategies (Watkins et al., 2014). Where necessary, parents themselves may also act as a peer model by participating in the mock training procedure as their child observed the process.
4.3. Limitations and future directions
In summary, core components implemented in the present MRI training protocol were as follows:
-
1
Pre-visit interview with parents to identify individualised reinforcers and preferred activities
-
2
Graduated exposure to stimuli encountered in scanner environment
-
3
Delivery of pre-visit scanner familiarisation materials by a familiar person
-
4
Peer-modelling with sibling involvement
-
5
Minimise identified triggers or aversive events in the mock scanning environment
-
6
High level of child engagement with parental involvement and effective communication
-
7
Consistent and predictable scheduling, in particular with the use of visual schedules
Essential aspects of the MRI training procedure were the implementation of a child-focused approach based on informant report, effective cohort-specific familiarisation strategies, and a collaborative effort with sibling and parents through peer modelling. While the sample size and minimal outcome measures may limit between-group comparisons and generalisability, an important consideration was to avoid unnecessary participant burden and fatigue. Although the inclusion of multiple measures of anxiety or adjustment levels pre- and post-training would ideally allow direct assessment of the efficacy of these proposed strategies, the tradeoff would likely come at the cost of excessive demands and burden on participants already engaged in a challenging procedure, and likely coping with symptom-related distress. This constraint forces selective and careful inclusion of measures and MRI sequences to those that are of higher priority to avoid over-burdening each child. For example, repeating an image acquisition sequence of key interest could be of more value than acquiring a range of different sequences. Based on our observations, a shorter study visit also appeared to be beneficial in minimising visit-related fatigue and improving the overall well-being of participants during the visit. We suggest that these factors could significantly impact scan performance, and recommend careful consideration when planning a research protocol.
The present sample likely comprised of higher functioning monozygotic twins with or without ASD, and were not compared on differences in exposure to familiarisation strategies. Future work in this area should further investigate the generalisability of these strategies across different low-functioning clinical populations with significant disability, as well as the potential for strategy modification depending on the nature and severity of disorder-specific symptoms that could impact image acquisition. While we have focused on evaluating image quality of task-free fMRI data, the efficacy of the proposed training on improving image acquisition could be further examined with controlled comparisons, and across different modalities. The influence of variability in individualised pre-scan training strategies on image acquisition should also be carefully considered as a potential source of systematic differences that could confound second-level analysis or group comparisons. Nevertheless, we suggest that the benefits of optimising the MRI training procedure for each child with a personalised child-focused approach outweigh the cost on time and resources, even in cases where head motion is likely to be satisfactory. In particular, disorder-specific and individualised strategies for neuroimaging children with neurodevelopmental conditions such as ASD are likely to minimise unnecessary distress and improve the overall well-being of each child during the MRI procedure, an experience which can be highly challenging even for typically developing children.
We found preliminary evidence that out-of-scanner head motion was associated with that observed in-scanner during the MRI scan. This secondary finding is consistent with recent efforts to mitigate the effects of head motion with real-time feedback during mock training, with mixed findings on whether head movement outside the scanner was generalisable to in-scanner head motion in MRI (Cox et al., 2017; Greene et al., 2018). The procedure involved training participants to reduce head movement by providing immediate visual feedback when head motion exceeds a certain threshold. Future work should further investiate the efficacy of such approaches in reducing in-scanner head motion. Together with individualised familiarisation and reinforcement strategies, these findings are promising and highlight the importance of a personalised child-focused approach to improve the quality of paediatric MRI image acquisition in challenging clinical populations such as ASD.
Declaration of Competing Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgements
This research was conducted within the Developmental Imaging research group, Murdoch Children’s Research Institute and the Children’s MRI Centre, Royal Children's Hospital, Melbourne, Victoria. It was supported by the Murdoch Children’s Research Institute, the Royal Children’s Hospital, Department of Paediatrics The University of Melbourne and the Victorian Government's Operational Infrastructure Support Program. The project was generously supported by RCH1000, a unique arm of The Royal Children’s Hospital Foundation devoted to raising funds for research at The Royal Children’s Hospital. This research was facilitated through access to Twins Research Australia, a national resource supported by a Centre of Research Excellence Grant (ID: 1079102), from the National Health and Medical Research Council. The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100750.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Blumenthal J.D., Zijdenbos A., Molloy E., Giedd J.N. Motion artifact in magnetic resonance imaging: implications for automated analysis. Neuroimage. 2002;16(1):89–92. doi: 10.1006/nimg.2002.1076. [DOI] [PubMed] [Google Scholar]
- Byars A.W., Holland S.K., Strawsburg R.H., Bommer W., Dunn R.S., Schmithorst V.J., Plante E. Practical aspects of conducting large-scale functional magnetic resonance imaging studies in children. J. Child Neurol. 2002;17(12):885–889. doi: 10.1177/08830738020170122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin J.B., Gurrin L.C., Sterne J.A., Morley R., Dwyer T. Regression models for twin studies: a critical review. Int. J. Epidemiol. 2005;34(5):1089–1099. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- Carr E.G., Dunlap G., Horner R.H., Koegel R.L., Turnbull A.P., Sailor W., Anderson J.L., Albin R.W., Koegel L.K., Fox L. Positive behavior support: evolution of an applied science. J. Posit. Behav. Interv. 2002;4(1):4–16. [Google Scholar]
- Carter A.J., Greer M.-L.C., Gray S.E., Ware R.S. Mock MRI: reducing the need for anaesthesia in children. Pediatr. Radiol. 2010;40(8):1368–1374. doi: 10.1007/s00247-010-1554-5. [DOI] [PubMed] [Google Scholar]
- Childress D.C. Special instruction and natural environments: best practices in early intervention. Infants Young Child. 2004;17(2):162–170. [Google Scholar]
- Cho Z., Park S., Kim J., Chung S., Chung S., Chung J., Moon C., Yi J., Sin C., Wong E. Analysis of acoustic noise in MRI. Magn. Reson. Imaging. 1997;15(7):815–822. doi: 10.1016/s0730-725x(97)00090-8. [DOI] [PubMed] [Google Scholar]
- Counter S.A., Olofsson A., Grahn H., Borg E. MRI acoustic noise: sound pressure and frequency analysis. J. Magn. Reson. Imaging. 1997;7(3):606–611. doi: 10.1002/jmri.1880070327. [DOI] [PubMed] [Google Scholar]
- Cox A.D., Virues-Ortega J., Julio F., Martin T.L. Establishing motion control in children with autism and intellectual disability: applications for anatomical and functional MRI. J. Appl. Behav. Anal. 2017;50(1):8–26. doi: 10.1002/jaba.351. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craske M.G., Treanor M., Conway C.C., Zbozinek T., Vervliet B. Maximizing exposure therapy: an inhibitory learning approach. Behav. Res. Ther. 2014;58:10–23. doi: 10.1016/j.brat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bie H.M.A., Boersma M., Wattjes M.P., Adriaanse S., Vermeulen R.J., Oostrom K.J., Huisman J., Veltman D.J., Delemarre-Van de Waal H.A. Preparing children with a mock scanner training protocol results in high quality structural and functional MRI scans. Eur. J. Pediatr. 2010;169(9):1079–1085. doi: 10.1007/s00431-010-1181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., O’Connor D., Chen B., Alaerts K., Anderson J.S., Assaf M., Balsters J.H., Baxter L., Beggiato A., Bernaerts S. Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Sci. Data. 2017;4 doi: 10.1038/sdata.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O., Birman D., Schaer M., Koyejo O.O., Poldrack R.A., Gorgolewski K.J. MRIQC: advancing the automatic prediction of image quality in MRI from unseen sites. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A.S., Whitfield-Gabrieli S., Roth R.M., Brunette M.F., Green A.I. Impaired functional connectivity of brain reward circuitry in patients with schizophrenia and cannabis use disorder: effects of cannabis and THC. Schizophr. Res. 2014;158(1-3):176–182. doi: 10.1016/j.schres.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gena A., Couloura S., Kymissis E. Modifying the affective behavior of preschoolers with autism using in-vivo or video modeling and reinforcement contingencies. J. Autism Dev. Disord. 2005;35(5):545–556. doi: 10.1007/s10803-005-0014-9. [DOI] [PubMed] [Google Scholar]
- Geschwind D.H., Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr. Opin. Neurobiol. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Greene D.J., Black K.J., Schlaggar B.L. Considerations for MRI study design and implementation in pediatric and clinical populations. Dev. Cogn. Neurosci. 2016;18:101–112. doi: 10.1016/j.dcn.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D.J., Koller J.M., Hampton J.M., Wesevich V., Van A.N., Nguyen A.L., Hoyt C.R., McIntyre L., Earl E.A., Klein R.L., Shimony J.S., Petersen S.E., Schlaggar B.L., Fair D.A., Dosenbach N.U.F. Behavioral interventions for reducing head motion during MRI scans in children. Neuroimage. 2018;171:234–245. doi: 10.1016/j.neuroimage.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagopian L.P., Jennett H.K. Behavioral assessment and treatment of anxiety in individuals with intellectual disabilities and autism. J. Dev. Phys. Disabil. 2008;20(5):467–483. [Google Scholar]
- Hallowell L.M., Stewart S.E., E Silva C.T.d.A., Ditchfield M.R. Reviewing the process of preparing children for MRI. Pediatr. Radiol. 2008;38(3):271. doi: 10.1007/s00247-007-0704-x. [DOI] [PubMed] [Google Scholar]
- Harvey M.T., Lewis-Palmer T., Horner R.H., Sugai G. Trans-situational interventions: generalization of behavior support across school and home environments. Behav. Disord. 2003;28(3):299–312. [Google Scholar]
- Hernandez P., Ikkanda Z. Applied behavior analysis: behavior management of children with autism spectrum disorders in dental environments. J. Am. Dent. Assoc. 2011;142(3):281–287. doi: 10.14219/jada.archive.2011.0167. [DOI] [PubMed] [Google Scholar]
- Horner R.H., Carr E.G., Strain P.S., Todd A.W., Reed H.K. Problem behavior interventions for young children with autism: a research synthesis. J. Autism Dev. Disord. 2002;32(5):423–446. doi: 10.1023/a:1020593922901. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson C.P., Myers S.M. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120(5):1183–1215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- Makowski C., Lepage M., Evans A.C. Head motion: the dirty little secret of neuroimaging in psychiatry. Journal of psychiatry & neuroscience: JPN. 2019;44(1):62. doi: 10.1503/jpn.180022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi A., DeMayo M.M., Glozier N., Guastella A.J. An overview of autism Spectrum disorder, heterogeneity and treatment options. Neurosci. Bull. 2017;33(2):183–193. doi: 10.1007/s12264-017-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E. arXiv Preprint arXiv:1704.01469. 2017. Notes on creating a standardized version of DVARS. [Google Scholar]
- Nordahl C.W., Mello M., Shen A.M., Shen M.D., Vismara L.A., Li D., Harrington K., Tanase C., Goodlin-Jones B., Rogers S., Abbeduto L., Amaral D.G. Methods for acquiring MRI data in children with autism spectrum disorder and intellectual impairment without the use of sedation. J. Neurodev. Disord. 2016;8:20. doi: 10.1186/s11689-016-9154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoe H.R., Hiess R.K., Kuzniecky R. Motion and morphometry in clinical and nonclinical populations. Neuroimage. 2016;135:177–185. doi: 10.1016/j.neuroimage.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Schlaggar B.L., Petersen S.E. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;105:536–551. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pua E.P.K., Bowden S.C., Seal M.L. Autism spectrum disorders: neuroimaging findings from systematic reviews. Res. Autism Spectr. Disord. 2017;34:28–33. [Google Scholar]
- Raschle N., Zuk J., Ortiz-Mantilla S., Sliva D.D., Franceschi A., Grant P.E., Benasich A.A., Gaab N. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann. N. Y. Acad. Sci. 2012;1252(1):43–50. doi: 10.1111/j.1749-6632.2012.06457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J.D., Luiselli J.K., Rue H., Whalley K. Graduated exposure and positive reinforcement to overcome setting and activity avoidance in an adolescent with autism. Behav. Modif. 2013;37(1):128–142. doi: 10.1177/0145445512456547. [DOI] [PubMed] [Google Scholar]
- Schneider N., Goldstein H. Using social stories and visual schedules to improve socially appropriate behaviors in children with autism. J. Posit. Behav. Interv. 2010;12(3):149–160. [Google Scholar]
- Shane H.C. Using visual scene displays to improve communication and communication instruction in persons with autism spectrum disorders. Augment. Altern. Commun. 2006;15(1) [Google Scholar]
- Shaw P., Stringaris A., Nigg J., Leibenluft E. Emotion dysregulation in attention deficit hyperactivity disorder. Am. J. Psychiatry. 2014;171(3):276–293. doi: 10.1176/appi.ajp.2013.13070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z., Giavasis S., Li Q., Benhajali Y., Yan C., Yang Z., Milham M., Bellec P., Craddock C. The preprocessed connectomes project quality assessment protocol—a resource for measuring the quality of MRI data. Front. Neurosci. 2015 [Google Scholar]
- Shivers C.M., Plavnick J.B. Sibling involvement in interventions for individuals with autism Spectrum disorders: a systematic review. J. Autism Dev. Disord. 2014;45(3):685–696. doi: 10.1007/s10803-014-2222-7. [DOI] [PubMed] [Google Scholar]
- Simonoff E., Pickles A., Charman T., Chandler S., Loucas T., Baird G. Psychiatric disorders in children with autism Spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Smyser C.D., Inder T.E., Shimony J.S., Hill J.E., Degnan A.J., Snyder A.Z., Neil J.J. Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratis E.A., Lecavalier L. Informant agreement for youth with autism Spectrum disorder or intellectual disability: a meta-analysis. J. Autism Dev. Disord. 2014;45(4):1026–1041. doi: 10.1007/s10803-014-2258-8. [DOI] [PubMed] [Google Scholar]
- Thapar A., Cooper M., Rutter M. Neurodevelopmental disorders. Lancet Psychiatry. 2017;4(4):339–346. doi: 10.1016/S2215-0366(16)30376-5. [DOI] [PubMed] [Google Scholar]
- Tonge B.J., Bull K., Brereton A., Wilson R. A review of evidence-based early intervention for behavioural problems in children with autism spectrum disorder. Curr. Opin. Psychiatry. 2014;27(2):158–165. doi: 10.1097/YCO.0000000000000043. [DOI] [PubMed] [Google Scholar]
- Trivette C.M., Dunst C.J., Sandall S. DEC Recommended Practices in Early intervention/early Childhood Special Education. 2000. Recommended practices in family-based practices; pp. 39–46. [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wåhlstedt C., Thorell L.B., Bohlin G. Heterogeneity in ADHD: neuropsychological pathways, comorbidity and symptom domains. J. Abnorm. Child Psychol. 2009;37(4):551–564. doi: 10.1007/s10802-008-9286-9. [DOI] [PubMed] [Google Scholar]
- Watkins L., O’Reilly M., Kuhn M., Gevarter C., Lancioni G.E., Sigafoos J., Lang R. A review of peer-mediated social interaction interventions for students with autism in inclusive settings. J. Autism Dev. Disord. 2014;45(4):1070–1083. doi: 10.1007/s10803-014-2264-x. [DOI] [PubMed] [Google Scholar]
- Wolpe J. Psychotherapy by reciprocal inhibition. Conditional reflex: a Pavlovian journal of research & therapy. 1968;3(4):234–240. doi: 10.1007/BF03000093. [DOI] [PubMed] [Google Scholar]
- Yerys B.E., Jankowski K.F., Shook D., Rosenberger L.R., Barnes K.A., Berl M.M., Ritzl E.K., Vanmeter J., Vaidya C.J., Gaillard W.D. The fMRI success rate of children and adolescents: typical development, epilepsy, attention deficit/hyperactivity disorder, and autism spectrum disorders. Hum. Brain Mapp. 2009;30(10):3426–3435. doi: 10.1002/hbm.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.