Table 1.
Reaction optimizationa,b.
| Entry | Photocatalysts | Solvent | Time (h) | Yieldb/% |
|---|---|---|---|---|
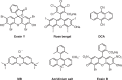
| 1 | Eosin Y | DMSO | 15 | 64 |
| 2 | Rose bengal | DMSO | 12 | 72 |
| 3 | Acridinium salt | DMSO | 36 | 64 |
| 4 | DCA | DMSO | 15 | NR |
| 5 | MB | DMSO | 15 | Trace |
| 6 | Ensin B | DMSO | 24 | 61 |
| 7 | Ru(bpy)3Cl2·6H2O | DMSO | 36 | 62 |
| 8 | fac-Ir(ppy)3 | DMSO | 36 | 56 |
| 9 | Rose bengal | DMF | 12 | 10 |
| 10 | Rose bengal | MeCN | 12 | 20 |
| 11 | Rose bengal | MeOH | 12 | 51 |
| 12 | Rose bengal | CHCl3 | 12 | Trace |
| 13 | Rose bengal | Acetone | 12 | 55 |
| 14 | Rose bengal | Toluene | 12 | NR |
| 15 | Rose bengal | EtOAc | 12 | 15 |
| 16 | Rose bengal | 1,4-dioxane | 12 | 22 |
| 17c | Rose bengal | DMSO | 12 | 51 |
| 18 | – | DMSO | 12 | NR |
| 19d | Rose bengal | DMSO | 12 | NR |
 | ||||
NR no reaction.
aThe reactions were carried out with 1a (0.2 mmol), CF2HSO2Na 2 (0.4 mmol), photocatalyst (2 mol%) in 1 mL solvent under two 3 W green LEDs irradiation at room temperature.
bIsolated yield.
cThe photocatalyst loading was decreased to 1 mol%.
dIn the dark.
