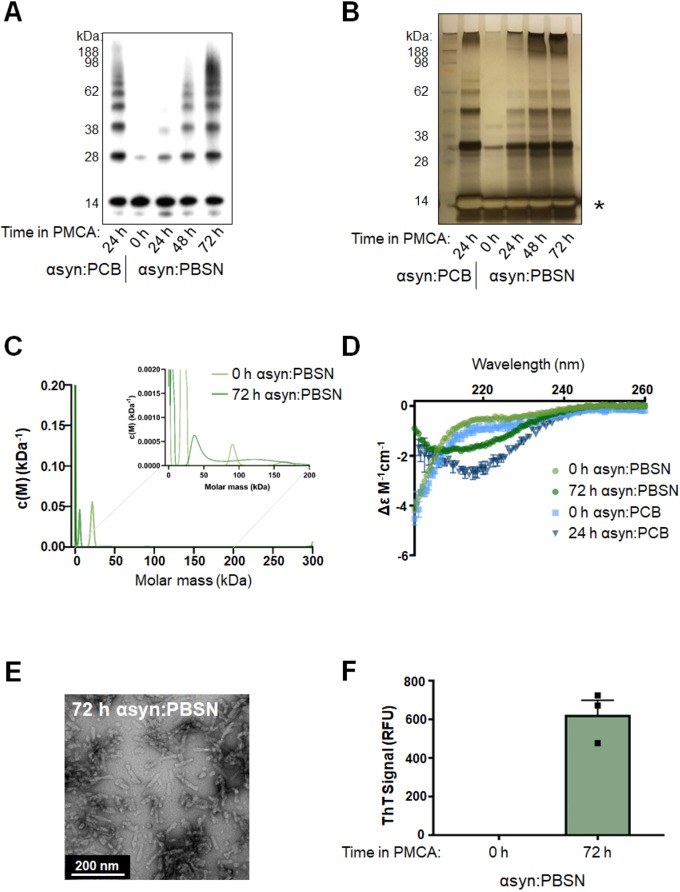
Fig. 2.
Production of misfolded α-synuclein species in PBSN buffer produces comparable misfolded content to α-synuclein species generated in PCB. Lyophilized recombinant wild-type protein was reconstituted in PBSN (α-synuclein:PBSN) or PCB (α-synuclein:PCB) and subjected to PMCA for a total process time of 0, 24, 48 or 72 h. (A,B) Extent of fibrillization in samples was assessed using (A) western immunoblot analysis using α-synuclein-specific monoclonal antibody MJFR1 (amino acid specificity: 118–123) and (B) silver staining, where the asterisk indicates saturation of the protein signal. (C) Analytical ultracentrifugation sedimentation velocity analyses of non-PMCA and PMCA-generated α-synuclein. Continuous mass distribution [c(M)] distributions are shown as a function of molar mass. Inset shows zoomed-in image of values between 0 and 200 kDa. Best fits to experimental data yielded a root mean square deviation of 0.0055 and 0.0045, f/f0 of 2.2 and 1.1, and Runs Test Z of 12 and 0.59 for non-PMCA and PMCA-generated α-synuclein, respectively. (D) Secondary structure of non-PMCA (0 h) or PMCA-generated (24 h or 72 h) α-synuclein:PBSN and α-synuclein:PCB was determined using circular dichroism (CD) spectroscopy (n=1, representative spectra). (E) TEM of fibrils produced in PBSN after 72 h PMCA (representative image). (F) α-Synuclein:PBSN exposed to PMCA for 72 h or non-PMCA (0 h) monomeric controls were further characterized by ThT fluorescence, where the values obtained for each experimental replicate represented the average fluorescence of triplicate wells after subtraction of a blank well to account for background fluorescence. RFU, relative fluorescent units. Data are presented as mean±s.e.m. (n=3) and represent every experimental replicate performed.