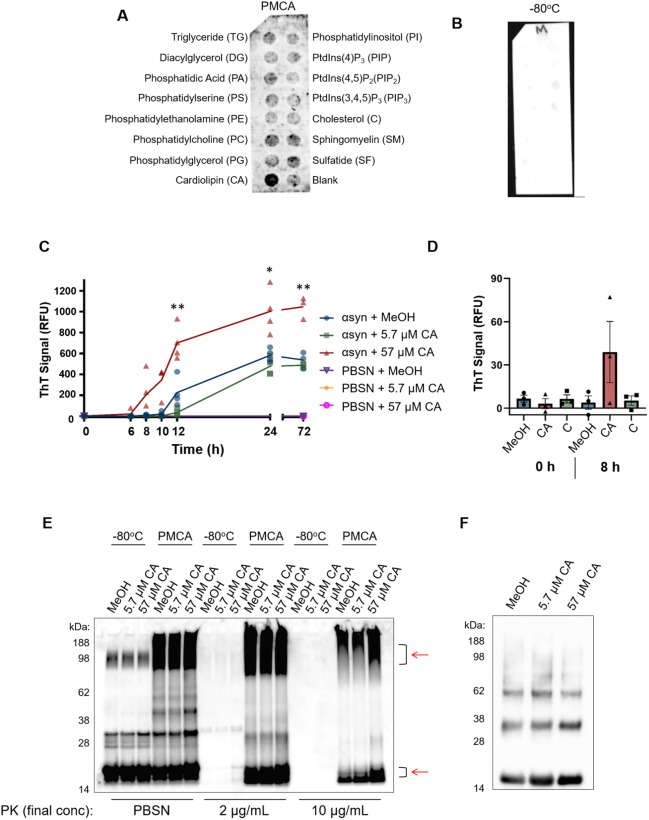
Fig. 3.
PMCA-generated α-synuclein selectively associates with cardiolipin. A hydrophobic membrane strip dotted with 15 different lipids (long-chain >diC16 synthetic analogues) was incubated with PMCA-generated α-synuclein. (A) Binding of α-synuclein to lipids was assessed via western immunoblotting using α-synuclein-specific monoclonal antibody MJFR1 (amino acid specificity: 118–123). (B) Unlike PMCA-generated misfolded α-synuclein, monomeric α-synuclein (−80°C) did not show affinity to any lipid class, n=1. To determine whether cardiolipin (CA) modulates α-synuclein fibrillization, CA was dissolved in MeOH/PBSN (MeOH) and added to wild-type α-synuclein to a final concentration of 5.7 μM and 57 µM CA. MeOH/PBSN alone added to α-synuclein:PBSN served to control for the effect of the solvent. (C) Samples were subjected to PMCA for a total process time of 0, 6, 8, 10, 12, 24 and 72 h. Tubes containing PBSN only (no α-synuclein) with solvent or CA exposed to PMCA for 0 h and 72 h served to detect any inherent fluorescence caused by CA or the solvent. Extent of fibrillization was assessed by measuring ThT fluorescence, where the values obtained for each experimental replicate represented the average fluorescence of triplicate wells after subtraction of a blank well to account for background fluorescence. RFU, relative fluorescent units. Data are presented as mean±s.e.m. (n=4 for all groups except for 0 h and 72 h, for which n=3) and represent every experimental replicate performed. To determine the effect of CA on α-synuclein fibrillization compared to control samples, a mixed-effects ANOVA with Dunnett's multiple comparisons test was employed. *P<0.05, **P<0.01. (D) ThT fluorescence was repeated in the presence of CA, MeOH or cholesterol (C), which showed no binding affinity to α-synuclein in panel A. ThT was measured after 8 h, which represented the earliest detectable rise in ThT by CA observed in panel C. The values obtained for each experimental replicate represented the average fluorescence of triplicate wells after subtraction of a blank well to account for background fluorescence. Data are presented as mean±s.e.m. (n=3) and represent every experimental replicate performed. (E) The biochemical properties of α-synuclein species formed after 72 h PMCA (PMCA) or not (−80°C) was assessed by its resistance to proteolysis following digestion in proteinase K (PK) at a final concentration of 0, 2 or 10 µg/ml. PK-resistant species were detected by immunoblotting using MJFR1. Areas of differential PK resistance between PMCA-generated species are identified by red arrows. n=1. (F) Ultracentrifugation was performed on α-synuclein in the presence of 5.7 µM and 57 µM CA or buffer control to determine the insoluble load. n=1.