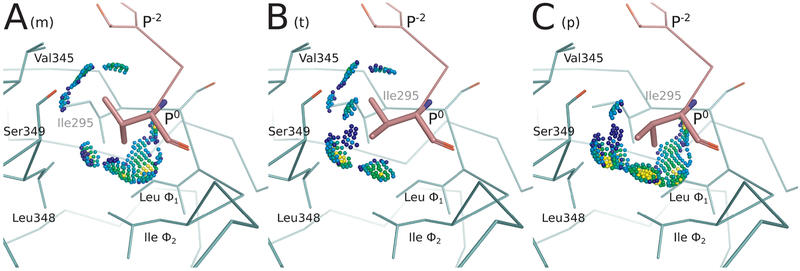
Figure 3.
Energy landscape analysis reveals conformational heterogeneity at Val P0 for CALP:kCAL01. Energy landscape analysis of bound kCAL01 indicates three rotamers at peptide P0 that contribute significantly to the partition function. We refer to these rotamers as m, t, or p, which describe the valine N–Cα–Cβ–Cγ1 dihedral angle as minus 60 (~−60°), trans (~180°), or plus 60 (~60°), respectively, conforming to the convention defined in ref 50. This landscape analysis (see Figure 4C, outermost ring) suggests that the complex can sample any of these rotamers with relatively high probability, but that the m rotamer will be most occupied, and the p rotamer will be least occupied. Conformations containing each of these three rotamers were selected from the bound kCAL01 ensemble. Interactions between the P0 side-chain atoms and the CALP structure are shown using Probe dots,78 where green and blue dots indicate favorable interactions, yellow dots indicate small overlaps, and red and pink lines show steric clashes. (A) The m rotamer forms favorable interactions with Thr P−2, Val345, Leu348, Ile Φ2, and Leu Φ1. (B) The t rotamer forms favorable interactions with Thr P−2, Val345, Ser349, Leu348, and Ile Φ2. The slight overlaps (yellow dots) generated due to the interaction with Leu348, along with the lack of interaction with Leu Φ1, suggest that this conformation is slightly less favorable than the m rotamer. (C) The p rotamer forms favorable interactions with Ser349, Leu348, Ile Φ2, and Leu Φ1. Slight overlaps (yellow dots) can be seen in interactions with Leu348, Ile Φ2, and Leu Φ1, and there is no interaction with Val345, suggesting that this rotamer may be slightly less favorable than either the m or t rotamers. Nevertheless, all three rotamers are well-sampled in the ensemble and contribute significantly to the partition function.