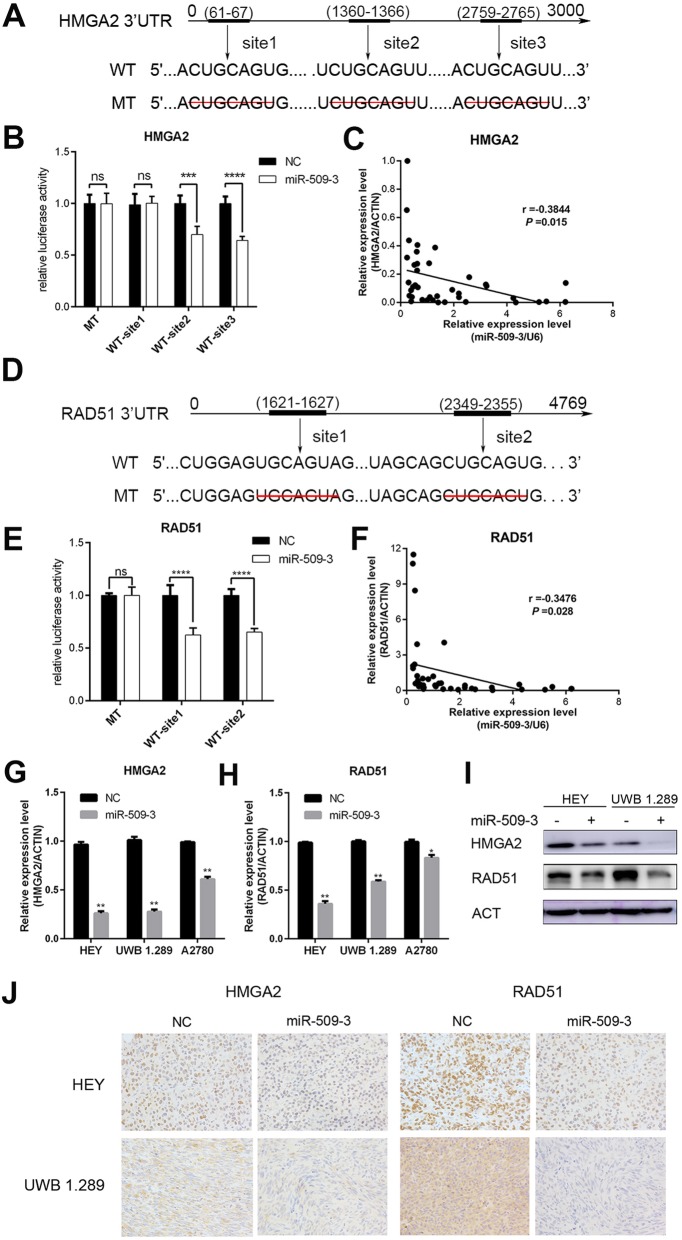
Fig. 4.
HMGA2 and RAD51 are direct targets of miR-509-3. a Putative binding sites in the HMGA2 3’UTR. There were three putative miR-509-3 binding sites and binding sequence was deleted as mutated type, which were cloned into pmirGLO vector respectively. b HEK293T cell was used in the Dual-Glo luciferase assay system. The luciferase activity of pmirGLO plasmid with wild-type (WT) HMGA2 3′UTR is weaker than that with mutant-type (MT) in HEK293T cell (site2, P < 0.001; site3, P < 0.0001). c Correlation between miR-509-3 and HMGA2. Relative HMGA2 mRNA level was negatively correlated with miR-509-3 level in RT-qPCR assay of tumor samples (Pearson r = − 0.3844, P = 0.015). d–f Exhibited the similar research toward RAD51 and concluded that miR-509-3 also directly targeted RAD51. g, h HMGA2 and RAD51 mRNA level in miR-509-3 overexpressing cells. Relative mRNA expressions were downregulated when cells were transfected with miR-509-3 mimics in A2780, HEY and UWB1.289. i Western blot assay demonstrated that miR-509-3 remarkably reduced protein expression level of HMGA2 and RAD51 in HEY and UWB1.289. h IHC representative image of subcutaneous tumors. The tumor tissues were fixed by formalin and cut into 4-μm-thick sections to be incubated by HMGA2 or RAD51 primary antibody. The expression of HMGA2 and RAD51 were both reduced by miR-509-3 in IHC images of HEY and UWB1.289