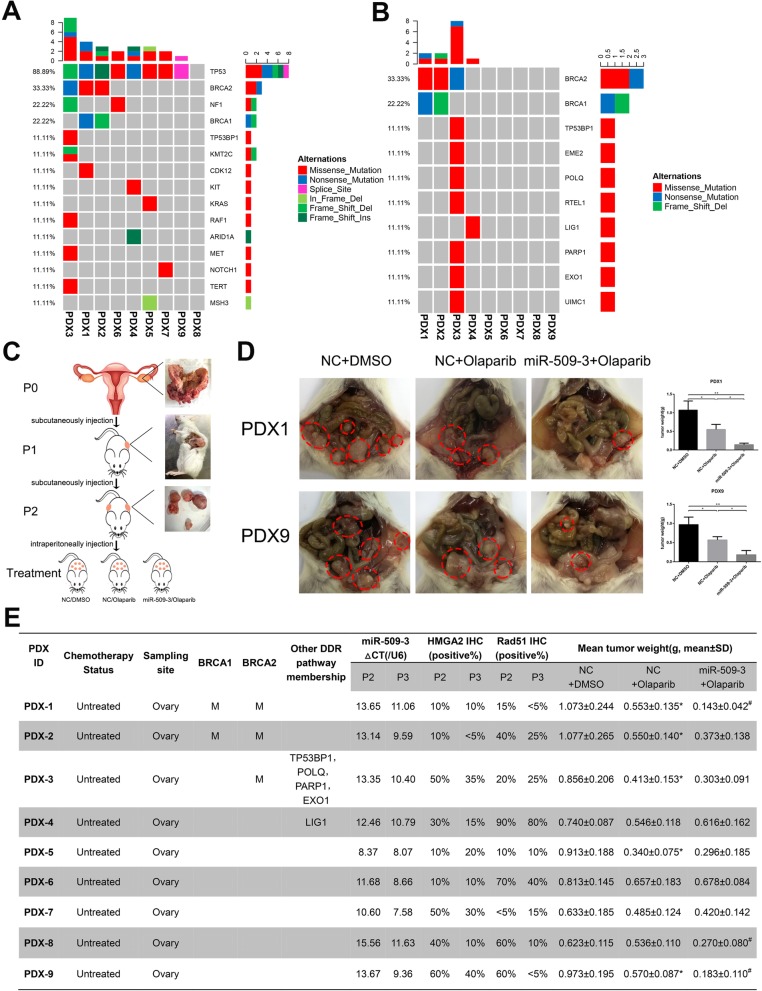
Fig. 6.
Genomic characterizations of the established PDX cases and Olaparib or miR-509-3 treatment responses in PDX models. a Ovarian cancer-driven gene mutation landscape of cases. Tumor and paired normal tissues of nine enrolled patients were sequenced by WES and 157 genes associated with ovarian and/or breast cancer susceptibility or tumorigenesis were annotated. The landscape of 9 PDX cases revealed the chief mutated genes in these HGSOC patients. The TP53 mutation rate is 88.89% (8/9). b HR pathway gene mutation landscape of cases. The landscape demonstrated that 44.44% (4/9) cases harbored HR gene mutation in which BRCA1/2 accounted for 75% (3/4). c The procedure of PDX model establishment and application in this study. P0 tumor tissues were obtained from gynecological surgery and implanted subcutaneously as P1. In the treatment groups (P3), AAV-miR-509-3 or AAV-NC were injected intraperitoneally twice within one week and then treated with Olaparib (50 mg/kg) once a day for 2 weeks. d Typical cases of miR-509-3 sensitization. PDX1 and PDX9 were representative cases in which miR-509-3 could enhance the sensitivity to Olaparib (mean tumor weights of NC + DMSO, NC + Olaparib, or miR-509-3 + Olaparib group respectively: PDX1, 1.073 ± 0.244, 0.553 ± 0.135, 0.143 ± 0.042; PDX9, 0.973 ± 0.195, 0.570 ± 0.087, 0.183 ± 0.110). Dotted red lines circled the tumor site in the abdomen of NCG mice. e The summarized list of detailed characteristics of each PDX cases including treatment status, sample origins, HR gene mutation status, endogenic miR-509-3 expression, HMGA2-positive rate, RAD51-positive rate, and mean tumor weight of three treatment groups (showed as means ± SD). In this figure, label “*” means this case is sensitive to Olaparib. Label “#” means miR-509-3 has sensitizing effect