Abstract
Empagliflozin, a SGLT-2 inhibitor, improves diabetic nephropathy through its pleiotropic anti-inflammatory effects. The present study aims to evaluate empagliflozin effects on renal and urinary levels of tubular epithelial cell injury markers in streptozotocin-induced diabetic rats. Empagliflozin at 10 mg/kg (p.o.) was administered for 4 weeks, beginning 8 weeks after induction of diabetes. Renal function as well as markers of renal tubular epithelial cell injury were assessed in kidney tissue homogenates and urine. Empagliflozin was able to ameliorate diabetes induced elevations in serum cystatin C levels. It also alleviated renal KIM-1/NGAL levels and urinary albumin, α-GST, and RBP excretions. In addition to decreasing urinary levels of cell cycle arrest indices i.e. TIMP-2 and IGFBP7, empagliflozin mitigated acetylated NF-κB levels in renal tissues of diabetic rats. As a whole, these findings reveal empagliflozin capability in improving diabetic nephropathy via ameliorating indices of renal inflammation, injury, and cell cycle arrest on streptozotocin-induced diabetic rats.
Keywords: Diabetic nephropathy, Empagliflozin, Tubular injury markers
Introduction
Diabetic nephropathy, the leading cause of end-stage renal disease (ESRD) across the world, imposes substantial medical costs annually associated with its progression to ESRD [1]. Over recent years, contribution of renal tubular epithelial cells to the pathogenesis of diabetic nephropathy have been recognized as they secrete increasingly amounts of inflammatory/fibrotic cytokines following their activation in diabetic milieu of the kidneys [2, 3]. Main culprits in activating renal tubular epithelial cells are excessive accumulation of intracellular glucose and unrestrained stimulation of receptors for advanced glycation end-products (AGEs) that culminate in hyperactivity of NF-κB signaling pathway, the master regulator of inflammation [4].
Currently, to ameliorate hallmarks of diabetic nephropathy i.e. renal inflammation/fibrosis and albuminuria, serum glucose and blood pressure control constitute the mainstay of therapy [5, 6]. Despite their promising outcomes, these measures do not consistently prevent initiation and progression of micro- and/or macrovascular complications of diabetes [7, 8]. Empagliflozin, a sodium-glucose cotransporter 2 (SGLT-2) inhibitor, is a FDA approved drug for the management of type 2 diabetes [9]. It mitigates glucose reabsorption in renal proximal tubules and thereby enhances urinary glucose excretions [10, 11]. In addition to glycemic control, empagliflozin provides nephroprotection by its direct pleotropic effects on the kidneys [12]. In like manner, protective potential of empagliflozin against progression of diabetic nephropathy have been documented in clinical trials [13].
Essentially, three categories of urinary biomarkers for early detection of renal tubular epithelial cell injuries have been recognized. Markers of inflammation constitute the first group; with interleukin 18 (IL-18) and neutrophil gelatinase-associated lipocalin (NGAL) being the most prominent ones. Second group are the cell injury markers and include principally kidney injury molecule 1 (KIM-1), liver fatty acid-binding protein (LFABP), and alpha glutathione S-transferase (α-GST). The third group includes cell cycle arrest markers like tissue inhibitor of metalloproteinase 2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP-7) [14, 15]. In the present investigation we aimed to evaluate effects of empagliflozin on renal and urinary markers of tubular dysfunction in streptozotocin-induced diabetic rats.
Materials and Methods
Chemicals
Empagliflozin was purchased from Cayman Chemical (Ann Arbor, Michigan, USA) and streptozotocin was obtained from SantaCruz (Dallas, Texas, USA).
Animal Experiments
Eight-week-old male Wistar rats (175–195 g) were obtained from Animal Care Center, Tabriz University of Medical Sciences. Housing conditions for all rats were the same including diet, temperature, humidity, light and dark cycles, and access to water. Experimental procedures were conducted in accordance with the instructions issued by the Council of Research and Technology, Tabriz University of Medical Sciences. Induction of diabetes was done by a single intraperitoneal (i.p.) injection of STZ (50 mg/kg) in 10 mM citrate buffer (pH 4.5) followed by a tail-blood glucometry 48 h after STZ injection to confirm diabetes; rats with blood glucose levels of 250 mg/dl or greater were considered diabetic. Animals were assigned into three group of: (1) healthy control rats (control) (n = 8); (2) diabetic control rats (Diab) (n = 8); and (3) empagliflozin treatment rats (10 mg/kg p.o.) [16]. Animals were housed in standard conditions for 2 month in order to allow renal sequelae of diabetes mellitus develop [17]; and then treatment with empagliflozin commenced for a period of 4 weeks. 12-h urine samples were collected by housing animals in metabolic cages at the penultimate day of the study. Finally, a lethal dose of 100 mg/kg ketamine and 1 mg/kg midazolam were injected; blood was collected via cardiac puncture; and kidneys were excised to histopathologic and chemical analyses.
Serum and Urine Immunochemical and Biochemical Analyses
Serum glucose and urine creatinine levels were assayed by using commercial kits (Pars Azmoon, Tehran, Iran). Blood hemoglobin A1c (HbA1c) was measured with an ion-exchange micro-column chromatography method using a commercial kit (BioSystems, Barcelona, Spain). A particle enhanced nephelometric immunoassay (PENIA) was adopted to measure serum cystatin C levels (GoldSite, Shenzhen, China). Urine albumin was measured by using a radio-immunoassay (RIA) kit (Immunotech, Prague, Czech). Urinary α-GST was assessed by a commercially available kit based on enzyme-linked immunosorbent assay (ELISA) technique (Aviva Bioscience, San Diego, California, USA). Urine TIMP-2 and IGFBP7 levels were evaluated by using chemiluminescent immunoassay (CLIA) kits. Finally, urine RBP levels were quantitated by a particle-enhanced turbidimetric immunoassay (PETIA) kit (Diazyme, Poway, California, USA). All values were normalized by urine creatinine levels.
Western Blotting
50 mg of kidney tissues were lysed in RIPA buffer (SantaCruz) and total protein content of the tissue homogenates were measured by the Bradford assay. After protein separation by SDS-PAGE, electro-blotting was performed to transfer protein bands onto PVDF membranes. Then membranes were blocked by non-fat skimmed milk solution followed by incubation in NGAL (SantaCruz, sc-515876), KIM1 (SantaCruz, sc-53769), and β-actin (SantaCruz, sc-47778) primary antibodies at 4 °C overnight. Thereafter, blots were incubated in HRP-labeled secondary antibodies at room temperature for 45 min and respective bands were visualized by Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Band densities on the pictures obtained from X-ray films were quantitated with ImageJ software (version 1.41) and normalized by the values obtained for β-actin bands as loading control.
For assessing acetylated NF-κB p65 levels in renal tissues, first nuclear fractions were separated by using a Nuclear Extraction Kit (Cayman Chemical, Ann Arbor, Michigan, USA); and then these nuclear extracts were run on SDS-PAGE in order to detect by western blotting. Primary acetylated NF-κB p65 antibody (Abcam, ab19870) was adopted along with NF-κB p65 primary antibody (SantaCruz, sc-8008) as the loading control. The remaining procedures were the same as described earlier.
Immunohistochemistry Examination
4–6 μm thick kidney tissue sections were mounted on glass slides and antigens enzymatically unmasked by 0.05% trypsin (Sigma-Aldrich, St. Louis, Missouri, USA) after deparaffinization step. Then for Immunohistochemical staining, tissue sections were incubated in blocking buffer (SantaCruz) for 1 h at room temperature followed by incubation with NGAL (SantaCruz, sc-515876), KIM1 (SantaCruz, sc-53769) primary antibodies overnight at 4 °C. Finally, the sections were incubated for 30 min in ready-to-use Avidin D-HRP (Santa Cruz, sc-516102) and color development was done by using DAB stains.
Statistical Analysis
Data are expressed as mean ± SD. One-way ANOVA followed by Tukey’s post hoc test was implemented for statistical comparisons; P < 0.05 was considered significant. The analyses were carried out using the SPSS software version 18 (IBM, Chicago, Illinois, USA).
Results
General Characteristics
General characteristics of study groups are shown in Table 1. In addition to weight lowering effects, treatment with empagliflozin resulted in decreased serum glucose and HbA1c levels in STZ-induced diabetic rats. Furthermore, diabetes induced elevations in serum cystatin C levels were effectively reduced after empagliflozin administration (Table 1).
Table 1.
General characteristics
| Control (n = 8) | Diab (n = 8) | EMP (n = 8) | |
|---|---|---|---|
| Body weight, g | 322.57 ± 12.26 | 279 ± 9.71a | 258 ± 9.14 |
| Serum glucose, mg/dl | 78.45 ± 3.31 | 414.93 ± 72.33a | 217 ± 52.87b |
| HbA1c, % | 5.1 ± 0.39 | 9.2 ± 0.91a | 6.3 ± 0.69b |
| Serum cystatin C, mg/dl | 1.55 ± 0.31 | 2.36 ± 0.67a | 1.92 ± 0.54b |
Values are presented as mean ± SD
Control healthy control rats, Diab diabetic control rats, EMP diabetic rats treated with Empagliflozin. HbA1c hemoglobin A1c
aP < 0.01 versus control rats
bP < 0.01 versus Diab rats
Empagliflozin Alleviates Renal Levels of KIM-1 and NGAL
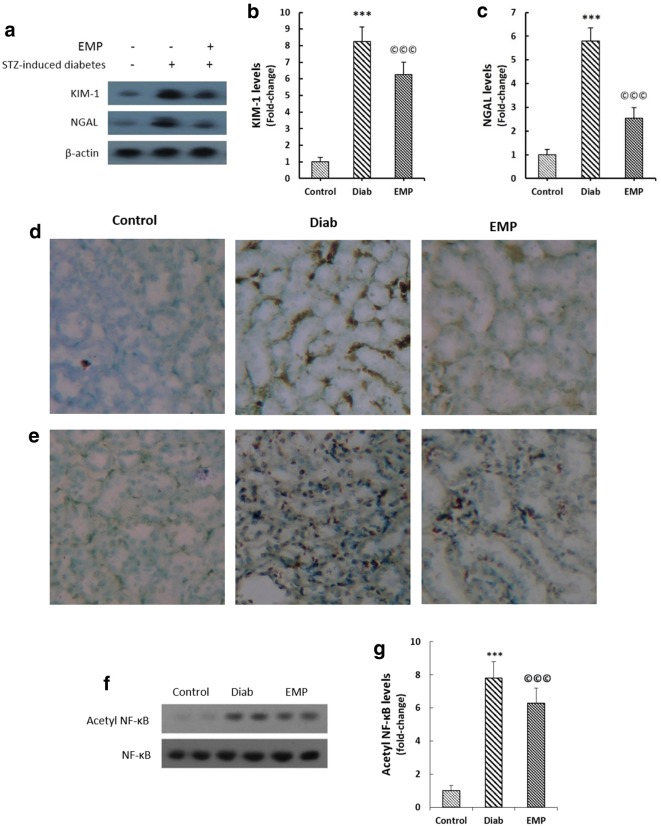
Renal expressions of KIM-1 and NGAL were evaluated by western blotting and immunohistochemical techniques as demonstrated in Fig. 1a–e. Diabetic rats exhibited significantly elevated levels of KIM-1 and NGAL expressions in their renal tissues. Empagliflozin efficiently mitigated their renal levels as indicated in Fig. 1. Additionally, significant reductions in acetylated NF-κB levels were observed in the kidney homogenates of diabetic rats after empagliflozin treatment (Fig. 1f, g).
Fig. 1.
Effects of EMP on renal KIM-1 (a, b, d), NGAL (a, c, e), and nuclear acetylated NF-κB p65 levels (f and g). a–c Representative bands of western blot together with semi-quantitative data for renal KIM-1 and NGAL. Data were normalized by the intensity of β-actin bands and then related to the values acquired by the controls. d, e Immunohistochemical staining of renal sections for KIM-1 and NGAL. f, g Renal acetylated NF-κB p65 levels. Nuclear fractions of tissue lysates were isolated by a nuclear extraction kit; total NF-κB p65 was adopted as the loading control. KIM-1 kidney injury molecule 1, NGAL neutrophil gelatinase-associated lipocalin, Control healthy control rats, Diab diabetic control rats, EMP diabetic rats treated with Empagliflozin. ***P < 0.01 versus Control rats, ©©©P < 0.01 versus Diab rats
Empagliflozin Ameliorates Urinary Indices of Renal Tubular Cell Injury
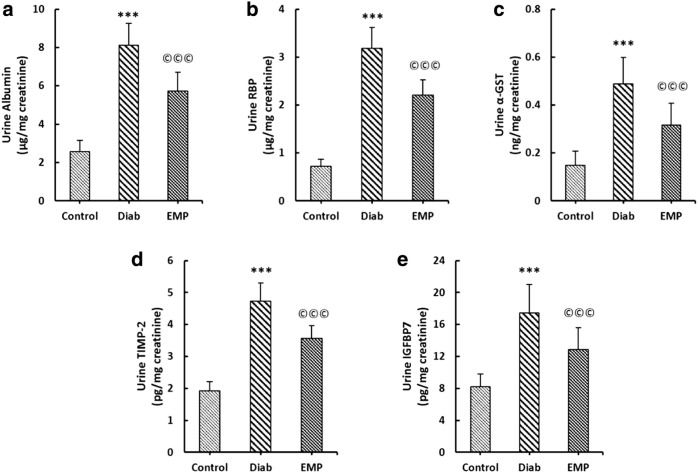
As depicted in Fig. 2, urinary levels of albumin, α-GST, RBP, TIMP-2, and IGFBP-7 were all significantly elevated in control diabetic rats. In addition to mitigating urinary levels of albumin, empagliflozin greatly decremented α-GAST, RBP, TIMP-2, IGFBP-7 levels in the urine of diabetic rats (Fig. 2).
Fig. 2.
Effects of EMP on urine albumin (a), RBP (b), α-GST (c), TIMP-2 (d), and IGFBP7 (e) levels in urine. All proteins were measured in 12-h urine samples obtained by housing animals in metabolic cages at the ultimate day of the study. All values were normalized by urinary creatinine concentrations. RBP retinol-binding protein, α-GST alpha glutathione S-transferase, TIMP-2 tissue inhibitor of metalloproteinase 2, IGFBP7 insulin like growth factor binding protein 7, Control healthy control rats, Diab diabetic control rats, EMP diabetic rats treated with Empagliflozin. ***P < 0.01 versus Control rats, ©©©P < 0.01 versus Diab rats
Discussion
In the present investigation we demonstrated for the first time that empagliflozin, in addition to ameliorating renal and urinary markers of tubular cell injury i.e. NGAL, KIM-1, α-GST, and RBP, effectively mitigated urinary excretions of TIMP-2 and IGFBP7, the indicators of cell cycle arrest, in STZ-induced diabetic rats. It further alleviated diabetes-induced elevations of renal acetylated NF-κB p65 levels and diminished serum levels of cystatin C, the sensitive indicator of kidney function [18].
Increased rates of renal tubular cell injuries results in cell cycle arrest to avoid division of cells with damaged DNA; in fact, cell cycle arrest is deemed to be a renoprotective mechanism [19]. In such circumstances, two cellular proteins including TIMP-2 and IGFBP7 are overexpressed in renal tubular epithelial cells, and accordingly are called the markers of cell cycle arrest [20]. Upraise of these proteins in the urine indicates that renal tubular cells are under stress [21]. In addition to being urinary biomarkers, TIMP-2 and IGFBP7 contribute to the pathogenesis of diabetic nephropathy, exacerbating renal fibrosis through modulating TGFβ-Smad signaling pathway [22, 23]. In the present investigation, we demonstrated elevated levels of urinary TIMP-2 and IGFBP7 in STZ rats; and further showed that empagliflozin effectively reduced both indices of cell cycle arrest in the urine.
KIM-1, NGAL, and LFABP has long been identified as sensitive markers of tubular damage; these proteins have a wide tissue distribution; however, their expressions are highly up-regulated after insult to renal tissues like diabetic nephropathy [24]. It has been found that 4-week EMP treatment successfully reduces urinary LFABP and albumin levels in STZ diabetic rats [16]. In agreement with these findings, we observed decreased renal expressions of KIM-1 and NGAL in STZ-induced diabetic rats. Supporting this, urinary levels of α-GST, the specific marker of renal proximal tubular epithelial cells damage [15], were also dampened in the present study. Contrary to the findings of Gallo et al. [11], that reported no reductions in urinary levels of KIM-1 and NGAL in db/db mice after a 10 week EMP therapy, we documented significant reductions in these two injury markers in renal tissues of diabetic rats. To confirm our findings, we also evaluated urinary RBP levels. RBP is a plasma protein with low molecular weight and therefore is freely filtered through renal glomeruli; a significant proportion of RBP is reabsorbed by renal tubular cells; however, in disease states, the malfunction in reabsorptive mechanisms leads to increased urinary RBP levels as the injury marker [25]. Empagliflozin was able to decrease notably urinary RBP levels in diabetic rats in the current investigation.
A wide array of findings accentuate that empagliflozin and other SGLT-2 inhibitors ameliorate diabetic kidney disease by alleviating renal inflammation and fibrosis mainly through down-regulation of NF-κB and TGFβ/Smad signaling pathways [11, 16, 26–28]. In favor of these findings, we assessed renal levels of acetylated NF-κB p65, the master regulator of inflammation, and found diminished levels of acetylated NF-κB p65 in the nuclear fractions of kidney tissue homogenates that highlights pleiotropic anti-inflammatory effects of empagliflozin.
Conclusion
Collectively, the findings of the present investigation underline nephroprotective effects of empagliflozin in streptozotocin-induced diabetic rats by the demonstration of reduced renal tubular epithelial cell injury markers principally α-GST, TIMP-2, and IGFBP7, improved kidney function test, and decremented acetylated NF-κB p65 levels. It had previously been known that empagliflozin confers protection against diabetic nephropathy, and in this study we shed more light on the protective effects of the agent.
Acknowledgements
This investigation is a supported by grants from Student Research center and Biotechnology Research Center, Tabriz University of Medical Sciences; and Urology and Nephrology Research Center, Beheshti University of Medical Sciences, Tehran, Iran.
Abbreviations
- SGLT-2
Sodium-glucose co-transporter 2
- KIM-1
Kidney injury molecule 1
- NGAL
Neutrophil gelatinase-associated lipocalin
- KIM-1
Kidney injury molecule-1
- α-GST
Alpha-glutathione S-transferase
- RBP
Retinol-binding protein
- TIMP-2
Tissue inhibitor of metalloproteinase 2
- IGFBP7
Insulin like growth factor binding protein 7
Compliance with Ethical Standards
Conflicts of interest
None of the authors declare any conflicts of interest.
References
- 1.Nichols GA, Vupputuri S, Lau H. Medical care costs associated with progression of diabetic nephropathy. Diabetes Care. 2011;34(11):2374–2378. doi: 10.2337/dc11-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 3.Tervaert TWC, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21(4):556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 4.Tang SC, Lai KN. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrol Dial Transplant. 2012;27(8):3049–3056. doi: 10.1093/ndt/gfs260. [DOI] [PubMed] [Google Scholar]
- 5.Gæde P, Lund-Andersen H, Parving H-H, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 6.Zoungas S, De Galan BE, Ninomiya T, Grobbee D, Hamet P, Heller S, et al. Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes. Diabetes Care. 2009;32(11):2068–2074. doi: 10.2337/dc09-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 8.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369(20):1892–1903. doi: 10.1056/NEJMoa1303154. [DOI] [PubMed] [Google Scholar]
- 9.Fitchett D, Butler J, van de Borne P, Zinman B, Lachin JM, Wanner C, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur Heart J. 2017;39(5):363–370. doi: 10.1093/eurheartj/ehx511. [DOI] [PubMed] [Google Scholar]
- 10.Škrtic M, Cherney DZ. Sodium–glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr Opin Nephrol Hypertens. 2015;24(1):96–103. doi: 10.1097/MNH.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 11.Gallo LA, Ward MS, Fotheringham AK, Zhuang A, Borg DJ, Flemming NB, et al. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci Rep. 2016;6:26428. doi: 10.1038/srep26428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R. SGLT2 inhibitors and the diabetic kidney. Diabetes Care. 2016;39(Supplement 2):S165–S171. doi: 10.2337/dcS15-3006. [DOI] [PubMed] [Google Scholar]
- 13.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 14.Lameire N, Vanmassenhove J, Van Biesen W, Vanholder R. The cell cycle biomarkers: promising research, but do not oversell them. Clin Kidney J. 2016;9(3):353–358. doi: 10.1093/ckj/sfw033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tesauro M, Nisticò S, Noce A, Tarantino A, Marrone G, Costa A, et al. The possible role of glutathione-S-transferase activity in diabetic nephropathy. Int J Immunopathol Pharmacol. 2015;28(1):129–133. doi: 10.1177/0394632015572564. [DOI] [PubMed] [Google Scholar]
- 16.Ojima A, Matsui T, Nishino Y, Nakamura N, Yamagishi S. Empagliflozin, an inhibitor of sodium-glucose cotransporter 2 exerts anti-inflammatory and antifibrotic effects on experimental diabetic nephropathy partly by suppressing AGEs-receptor axis. Horm Metab Res. 2015;47(09):686–692. doi: 10.1055/s-0034-1395609. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Chen H, Liu Q, Ma Q. Effect of simvastatin on the expression of nephrin, podocin, and vascular endothelial growth factor (VEGF) in podocytes of diabetic rat. Int J Clin Exp Med. 2015;8(10):18225. [PMC free article] [PubMed] [Google Scholar]
- 18.Randers E, Kristensen H, Erlandsen EJ, Danielsen S. Serum cystatin C as a marker of the renal function. Scand J Clin Lab Investig. 1998;58(7):585–592. doi: 10.1080/00365519850186210. [DOI] [PubMed] [Google Scholar]
- 19.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11(5):264. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashani K, Kellum JA. Novel biomarkers indicating repair or progression after acute kidney injury. Curr Opin Nephrol Hypertens. 2015;24(1):21–27. doi: 10.1097/MNH.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 21.Kellum JA. Diagnostic criteria for acute kidney injury: present and future. Crit Care Clin. 2015;31(4):621–632. doi: 10.1016/j.ccc.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe J, Takiyama Y, Honjyo J, Makino Y, Fujita Y, Tateno M, et al. Role of IGFBP7 in diabetic nephropathy: TGF-β1 induces IGFBP7 via Smad2/4 in human renal proximal tubular epithelial cells. PLoS ONE. 2016;11(3):e0150897. doi: 10.1371/journal.pone.0150897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X, Xiao L, Xiao P, Yang S, Chen G, Liu F, et al. A glimpse of matrix metalloproteinases in diabetic nephropathy. Curr Med Chem. 2014;21(28):3244–3260. doi: 10.2174/0929867321666140716092052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tramonti G, Kanwar YS. Tubular biomarkers to assess progression of diabetic nephropathy. Kidney Int. 2011;79(10):1042–1044. doi: 10.1038/ki.2011.9. [DOI] [PubMed] [Google Scholar]
- 25.Titan S, Vieira J, Dominguez W, Moreira S, Pereira A, Barros R, et al. Urinary MCP-1 and RBP: independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. J Diabetes Complicat. 2012;26(6):546–553. doi: 10.1016/j.jdiacomp.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Gembardt F, Bartaun C, Jarzebska N, Mayoux E, Todorov VT, Hohenstein B, et al. The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am J Physiol Renal Physiol. 2014;307(3):F317–F325. doi: 10.1152/ajprenal.00145.2014. [DOI] [PubMed] [Google Scholar]
- 27.Terami N, Ogawa D, Tachibana H, Hatanaka T, Wada J, Nakatsuka A, et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE. 2014;9(6):e100777. doi: 10.1371/journal.pone.0100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panchapakesan U, Pegg K, Gross S, Komala MG, Mudaliar H, Forbes J, et al. Effects of SGLT2 inhibition in human kidney proximal tubular cells—renoprotection in diabetic nephropathy? PLoS ONE. 2013;8(2):e54442. doi: 10.1371/journal.pone.0054442. [DOI] [PMC free article] [PubMed] [Google Scholar]