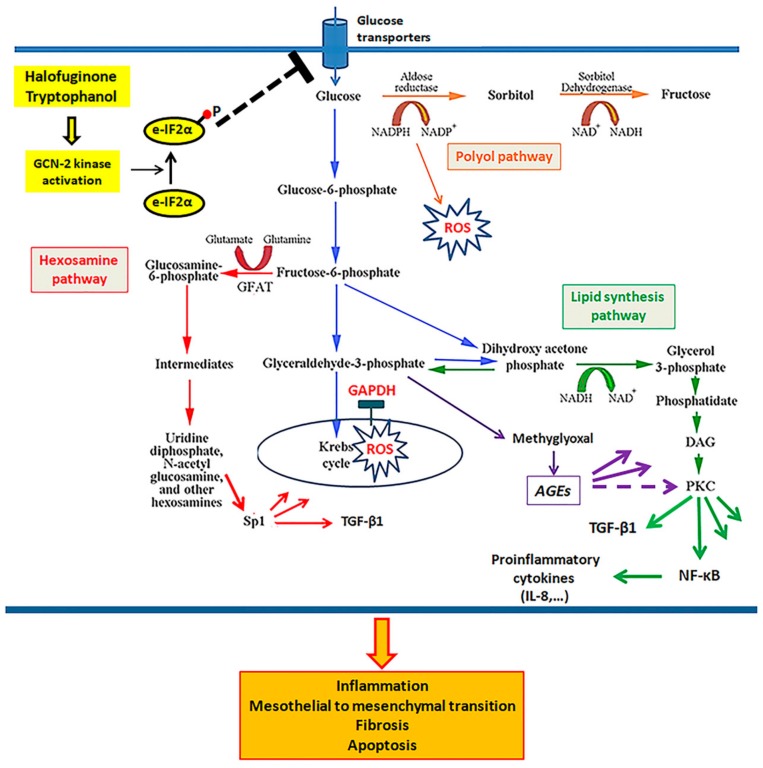
Figure 8.
The effect of GCN-2 kinase activation on glucotoxicity, cell integrity, and the mesothelial to mesenchymal transition in human peritoneal mesothelial cells. Under high-glucose conditions, glucose influx into mesothelial cells increases, likely due to the upregulation of glucose transporters. The elevated intracellular glucose is catabolized through the Krebs cycle and produces more electron donors (NADH and FADH2) than the oxidative phosphorylation chain has capacity to transfer electrons. The extra electrons convert molecular oxygen to superoxide. ROS inhibits the enzyme GAPDH, leading to accumulation of upstream glycolytic products, which are diverted to four noxious pathways. The polyol pathway utilizes NADPH, required for the reduction of glutathione. The hexosamine pathway results in the O-GlcNAc-modification of proteins, altering their function. For instance, the modification of Sp1 increases the transcription of the gene for the profibrotic TGF-β1. The lipid synthesis pathway produces DAG, an activator of PKC kinase able to activate NF-κB, known for its role in the expression of pro-inflammatory cytokines and TGF-β1. Finally, glyceraldehyde 3-phosphate is converted to MGO, a precursor of AGEs. All these glucotoxic events increase the production of the profibrotic TGF-β1 and pro-inflammatory cytokines, such as IL-8, and induce cell apoptosis and the mesothelial to mesenchymal transition—alterations involved in the peritoneal membrane pathology observed in PD patients with ultrafiltration failure. Activation of GCN-2 kinase by downregulating the expression of glucose transporters and glucose influx into the mesothelial cells prevents all of the above described glucotoxic pathways, preserves cell integrity, and prevents mesothelial to mesenchymal transition.