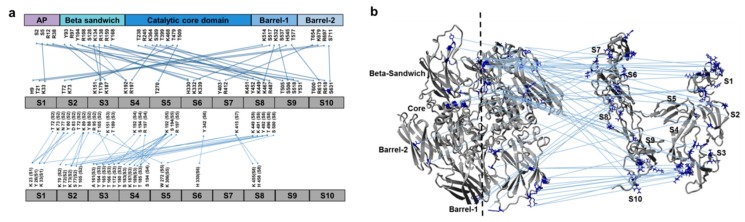
Figure 2.
Cross-linking and mass spectrometry (XL-MS) derived cross-linking residues of FXIII complex reveals an N-to C-terminal symmetry. (a) shows the domain-wise distribution of both FXIII-B monomer model docking to FXIII-A2 crystal structure, distance constraints (upper part of image), and monomer FXIII-B model assembly, distance constraints (lower part of image), that were generated from the XL-MS cross-linking information of the purified FXIII-A2B2 heterotetramer complex (Supplementary Tables S2 and S3). (b) shows a structural description of the information shown in (a). The crystal structure of the FXIII-A subunit dimer and the monomer model of the FXIII-B subunit have been illustrated in ribbon format.