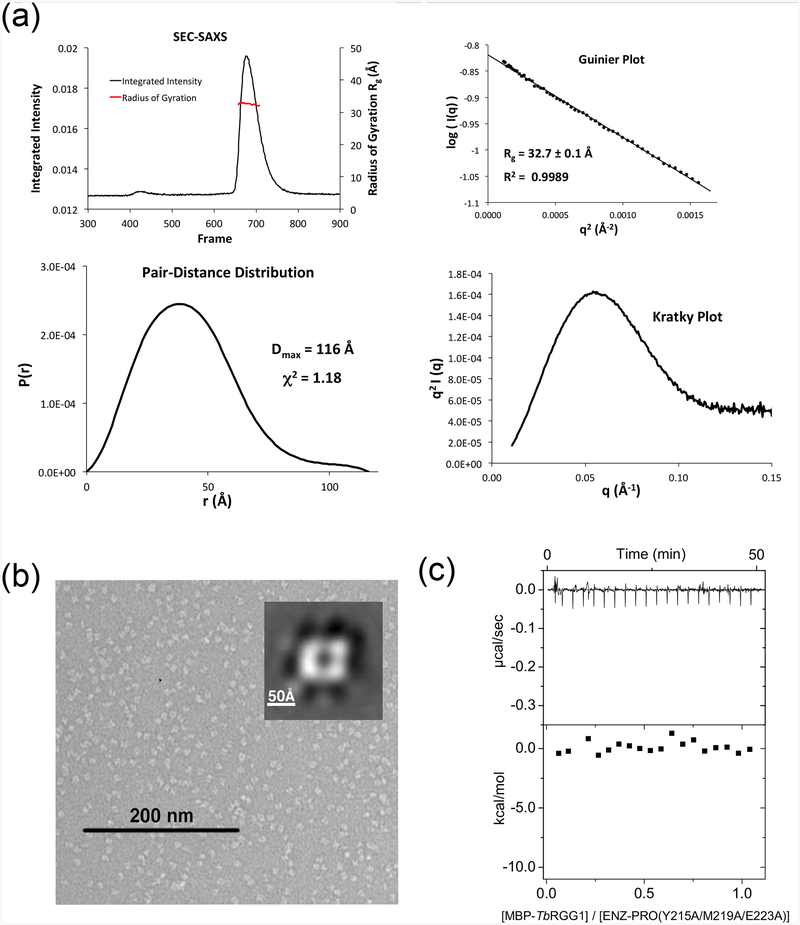
Figure 6. Structure-based mutagenesis of the tetrameric interface yields stable TbPRMT1 ENZ-PRO heterodimers.
(a) SEC–SAXS analysis of a representative heterodimeric TbPRMT1 ENZ-PRO mutant (the ENZ W46A/R47A/Y50A triple mutant). Top left panel: SEC-SAXS integrated intensities (left y-axis) plotted against frame number (x-axis). The red dots indicate radius of gyration, Rg (on the right y-axis). Top right panel: Guinier plot calculated from averaging buffer-subtracted scattering intensities. The coefficient of determination, R2, is 0.9989. Bottom left panel: Pair-distance distribution function P(r), yielding a maximum molecular diameter of 116 Å. Bottom right panel: Normalized Kratky plot calculated from SEC–SAXS data.
(b) Negative-stain electron microscopy analysis of a representative TbPRMT1 ENZ-PRO mutant (the PRO Y215A/M219A/E223A triple mutant). EM micrograph with a 200 nm scale bar. Inset: Predominant 2D class average.
(c) ITC thermogram (upper panel) and plot of corrected heat values (lower panel) for binding of the TbPRMT1 ENZ Y215A/M219A/E223A triple mutant to Maltose Binding Protein (MBP)-fused TbRGG1 protein.