Abstract
Background
The objective of this study is to review our 12-year experience with the 5-α reductase inhibitor dutasteride as a potential long-term treatment option for stuttering priapism. Dutasteride has a uniquely long half-life of 35 days which offers a theoretical advantage as a chronic therapy for management of stuttering priapism.
Methods
We retrospectively reviewed patients with stuttering priapism in our database from 2006–2018 treated with dutasteride. Men with concurrent use of medications other than dutasteride to treat stuttering priapism were excluded. Patients were started on a dose of 0.5 mg daily and tapered to a more infrequent dosing schedule, ranging from 0.5 mg every other day to once weekly. The frequency of priapism episodes before and after initiation of dutasteride therapy was analyzed.
Results
Among 21 cases, 13 patients met our inclusion criteria (mean age 43 years). Median follow-up on daily dutasteride was 79 days, and median follow-up on tapered dutasteride was 607 days. A total of 11/13 (85%) men treated with dutasteride had some degree of improvement—5/13 (38%) had complete resolution of their symptoms and 6/13 (46%) had reduced frequency and/or severity of their episodes. Among 5/13 (38%) men who had >2 emergency room (ER) visits for ischemic priapism prior to therapy, most (3/5, 60%) did not require any ER visits while on dutasteride therapy. Among the five men who received chronic, tapered-dose therapy, all reported continued suppression of priapistic episodes. Among 4 patients with sickle cell disease (SCD), 3/4 (75%) ultimately chose more invasive therapy including androgen deprivation therapy (ADT) and penile prosthesis. Side effects were minimal and included gynecomastia (8%), decreased libido (8%), and fatigue (8%).
Conclusions
In patients with stuttering priapism, daily dutasteride therapy is a promising treatment option to reduce the frequency and severity of priapistic episodes without significant side effects. Therapy can effectively be tapered to once weekly dosing without a reduction in efficacy.
Keywords: Erectile dysfunction, priapism, sick cell disease
Introduction
Recurrent ischemic priapism (RIP), also known as stuttering priapism, is a rare entity with poorly understood pathophysiology (1). Stuttering priapism is most commonly described in patients with sickle cell disease (SCD), in which the incidence has been reported to be as high as 64% (2-4). In adults, stuttering priapism is usually idiopathic, but other reported etiologies include medications, hematological disorders, neurological disorders, metabolic disorders, or neoplasms (5). Although many episodes of stuttering priapism can be self-limited, some progress to major ischemic events that require surgical intervention in the emergency room (ER) or operating room. These ischemic episodes need prompt intervention to prevent permanent vascular damage and corporal fibrosis, highlighting the need for effective preventative treatment options.
While conventionally used in the treatment of benign prostate hyperplasia (BPH), the 5-α reductase inhibitor (5ARI) finasteride has already been shown to be efficacious for treating stuttering priapism (6-10). Finasteride blocks the 5-α reductase (5AR) enzyme that converts testosterone (T) to dihydrotestosterone (DHT). Dutasteride is a similar 5ARI that has several features that make it attractive as a potential preventative treatment option for men with RIP. Finasteride inhibits only the type II isoform of the 5AR enzyme, while dutasteride is a dual 5ARI that blocks both type I and type II 5AR isoforms, producing more complete reduction of DHT (11,12). In comparison to finasteride, which has a half-life of approximately 5 hours, dutasteride’s uniquely long half-life of 35 days gives it a theoretical advantage as a chronic therapy in this setting.
To our knowledge, evidence for the effectiveness of dutasteride in the management of stuttering priapism is scarce. While it is unknown whether dutasteride is ultimately more effective or has a more pronounced effect than finasteride, dutasteride is preferred due to its longer half-life and greater reduction of circulating DHT. The objective of our study is to report our 12-year experience with dutasteride as a long-term preventative treatment option for patients with stuttering priapism.
Methods
We retrospectively reviewed our database of outpatient encounters from 2006–2018 at our tertiary referral center urology clinic to identify patients with stuttering priapism treated with dutasteride. Exclusion criteria included concurrent use of other medications for treatment of stuttering priapism. Patients were started on a dose of 0.5 mg daily and then tapered to a more infrequent dosing schedule, ranging from 0.5 mg every other day to 0.5 mg once weekly. Primary outcomes analyzed were the frequency of stuttering priapism episodes before and after therapy, as well as the number of ER visits required for priapism before and after therapy. Chi-square analysis was used to compare SCD (n=4) and non-SCD (n=9) patients.
Results
Among 21 cases, 13 patients met our inclusion criteria with a mean age of 43 years (range, 20–60 years) and median follow-up of 79 days (range, 18–491 days) on daily dutasteride (Table 1). A total of 5 patients were placed on tapered therapy with a median follow-up of 607 days (range, 90–1,095 days). The lead time to treatment with dutasteride was available for 10/13 (77%) patients with a median of 122 days.
Table 1. Patient characteristics.
| Patient No. | Age | Etiology | No. of ER aspirations prior | No. of shunt procedures in or prior | Prior medical therapies | No. of ER visits on dutasteride | Tolerated tapered therapy? | Side effects? |
|---|---|---|---|---|---|---|---|---|
| 1 | 29 | Sickle cell disease (SCD) | 1 | 0 | Unknown | 0 | Did not try | |
| 2 | 34 | SCD | Multiple | 0 | Terbutaline, self-phenylephrine injection | 1 | Did not try | |
| 3 | 37 | SCD | 2 | 0 | Lupron, pseudoephedrine | 0 | Did not try | |
| 4 | 38 | SCD | 0 | 0 | Lupron | 0 | Did not try | |
| 5 | 20 | Idiopathic | 3 | 0 | None | 2 | Yes | |
| 6 | 42 | Idiopathic | 0 | 0 | Lupron | 0 | Yes | |
| 7 | 45 | Idiopathic | 8 | 1 | None | 0 | Yes | Decreased libido |
| 8 | 51 | Idiopathic | 2 | 0 | None | 0 | Yes | |
| 9 | 60 | Idiopathic | 0 | 0 | Unknown | 0 | Did not try | Gynecomastia |
| 10 | 48 | Idiopathic | 1 | 1 | None | 0 | Yes | |
| 11 | 54 | Idiopathic | 1 | 2 | None | 0 | Did not try | |
| 12 | 56 | Idiopathic | 1 | 1 | None | 0 | Did not try | |
| 13 | 46 | Polycythemia | 1 | 1 | Self-phenylephrine injection | 0 | Did not try | Fatigue |
A total of 11/13 (85%) men treated with dutasteride had some degree of improvement—5/13 (38%) had complete resolution of their symptoms and 6/13 (46%) had reduced frequency and/or severity of their episodes (Figure 1). Of the two men who did not experience improvement, one experienced multiple painful erections daily and ultimately progressed to complete androgen deprivation while the other ultimately elected for penile prosthesis placement. A greater percentage of SCD patients progressed to more aggressive treatment, including ADT and penile prosthesis, compared to non-SCD patients (3/4, 75% vs. 2/9, 22%; P=0.07, Figure 2). However, there was no statistically significant difference in improvement between the two groups (3/4, 75% vs. 8/9, 89%; P=0.52, Figure 3).
Figure 1.
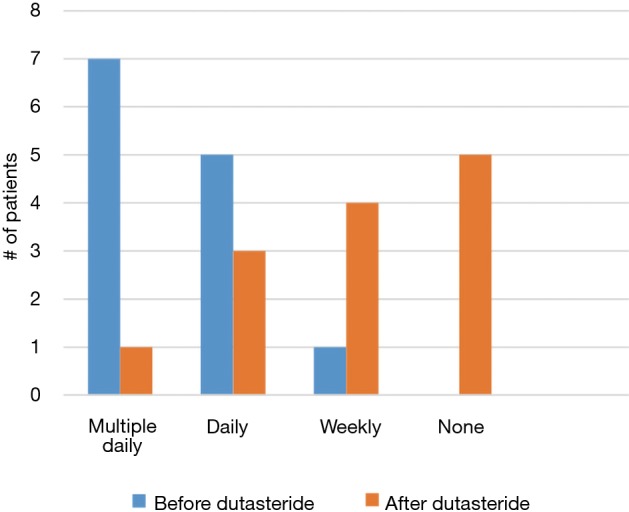
Frequency of stuttering priapism before and after dutasteride therapy.
Figure 2.
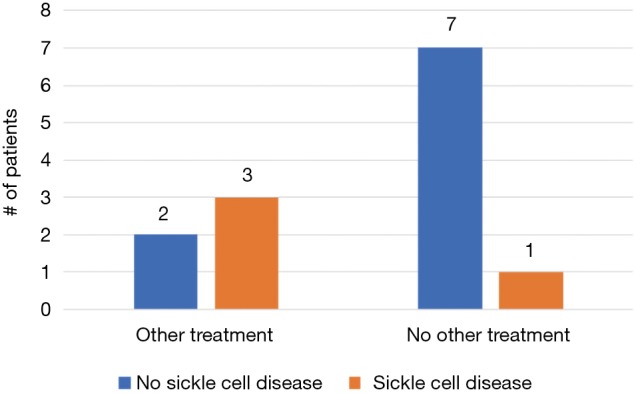
Comparison of progression to more aggressive treatment between sickle cell disease (SCD) and non-SCD patients.
Figure 3.
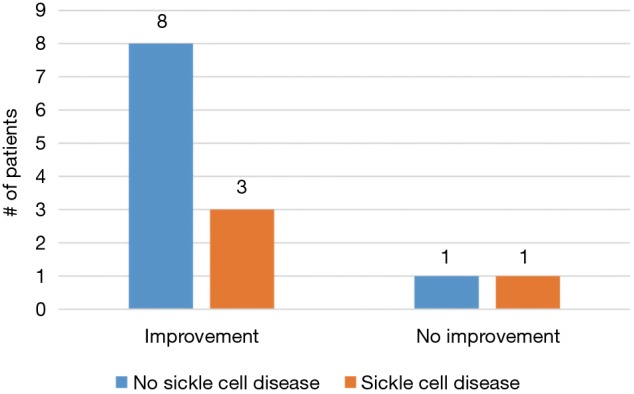
Comparison of improvement between sickle cell disease (SCD) and non-SCD patients.
A total of 10/13 (77%) required at least one ER visit for priapism prior to therapy. Among high risk patients (>2 ER visits prior to therapy, n=5), most (3/5, 60%) did not require any ER visits while on dutasteride therapy. One man required 2 ER visits for priapism less than 1 month after initiating dutasteride therapy, but ultimately had complete resolution of his symptoms and was tapered to twice weekly dosing without issues. Another was a SCD patient that required 1 ER visit on dutasteride and ultimately progressed to ADT, although he did note a decrease in painful erections from daily to weekly with dutasteride alone.
The five men that received chronic, tapered-dose therapy reported continued suppression of priapistic episodes. Side effects of dutasteride were minimal and experienced by only three patients, including gynecomastia (1/13, 8%), decreased libido (1/13, 8%), and fatigue (1/13, 8%). In this group, one patient discontinued therapy secondary to side effects.
Discussion
To our knowledge, this is the first paper demonstrating the efficacy of dutasteride as a chronic therapy for the prevention of stuttering priapism. While dutasteride has been shown to be a well-tolerated treatment in men with BPH, a review of the literature on stuttering priapism treatment over the past several decades reveals scant evidence on the use of the use of dutasteride (13). The majority of evidence on preventative treatment options for RIP is based on case-series and case reports, limiting the ability to make definitive recommendations on ideal treatment options for men with stuttering priapism.
Pathophysiology of priapism
The mechanism of stuttering priapism remains poorly understood. However, there have been recent advances in deciphering its pathophysiology. Studies suggest that RIP is a multifactorial process in which an imbalance occurs between normal erection and detumescence mechanisms. Deficiencies in endothelial nitric oxide (NO) have been shown to cause downregulation of PDE-5 and the subsequent inability to regulate cGMP, resulting in disproportionate responses to stimuli (14). Androgens have been shown to be important regulators of nitric oxide synthase (NOs) and PDE-5 in penile tissue, providing a potential explanation as to why anti-androgen therapies are effective in this setting (15,16).
Current treatment options
Numerous preventative treatment options for stuttering priapism have been reported with mixed efficacy, including α agonists, digoxin, baclofen, terbutaline, ketoconazole, gabapentin, phosphodiesterase 5 (PDE-5) inhibitors, and hormonal therapies (17-20). In the current literature, gonadotropin-releasing hormone (GnRH) agonists and antiandrogens have been common treatments of stuttering priapism. However, these treatments carry the potential for significant side effect profiles such as hot flashes, osteoporosis, and cardiovascular disease (21).
Hoeh and Levine demonstrated the advantages of ketoconazole, an antifungal drug that inhibits androgen production, in RIP treatment (20). They showed ketoconazole to be a safe, effective, well-tolerated, and relatively inexpensive treatment option for men with RIP. Additional advantages include its rapid effect on decreasing testosterone while preserving sexual function. One disadvantage of ketoconazole in this setting is the necessary concurrent administration of steroids in order to prevent adrenal insufficiency. We abandoned ketoconazole as a RIP management strategy after observing poor effectiveness and in an effort to avoid the need for steroid replacement.
Stuttering priapism occurs in a particularly young and active patient population, highlighting the need for preventative therapies with minimal side effects that preserve sexual function and fertility. The side effect profile in our study was minimal, with only one patient discontinuing therapy because of fatigue. This is a significant advantage of dutasteride compared to many other therapies commonly used in this setting.
5-α reductase inhibitors
It has been previously established that DHT plays an important role in promoting erectile function (15,22). The 5ARI finasteride reduces serum DHT levels by 70% and has been shown to be efficacious in the prevention of stuttering priapism in two studies (9,10). The larger of these studies, a series of 35 SCD patients started on 5 mg of finasteride daily and tapered to 1 mg daily, reported that 46% of their patients had no priapism recurrences after 120 days (10). Our experience with dutasteride was comparable, with 85% of patients having some degree of improvement and 38% reporting complete resolution of their symptoms with a median follow-up of 79 days.
Although our study was not adequately powered to detect significant differences in outcomes between SCD and non-SCD patients, it appears that dutasteride may be more efficacious in non-SCD patients. Most SCD patients [3/4 (75%)] progressed to more aggressive treatment options compared to 2/9 (22%) of non-SCD patients. This may indicate that SCD patients have a more severe disease process or different underlying mechanism to their recurrent priapism episodes.
Our data suggest that dutasteride therapy can be tapered to once weekly dosing without a reduction in efficacy, with no men reporting an increase in the number of stuttering episodes over a median follow-up of 607 days. In our experience, dutasteride is well-tolerated with fewer side effects than other treatment options. These advantages combined with the reduction of treatment cost with tapered therapy suggest that dutasteride is a promising therapy option for men with stuttering priapism.
Limitations
There are admittedly limitations to our study, including its retrospective analysis, small sample size, lack of control arm, and lack of comparison arms to other treatment modalities. Stuttering priapism is a rare disease process overall, and multi-center, randomized, prospective trials are ultimately needed to determine the effectiveness of dutasteride in comparison with placebo and other treatment options.
Conclusions
Dutasteride therapy is a promising treatment option for men with stuttering priapism to reduce the frequency and severity of priapistic episodes with only minimal side effects. Daily therapy can be tapered to once weekly dosing while maintaining efficacy.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of The University of Texas Southwestern Medical Center at Dallas (ID: 052011-161).
Footnotes
Conflicts of Interest: Dr. AF Morey receives honoraria for being a guest lecturer/meeting participant for Boston Scientific and Coloplast Corp. The other authors have no conflicts of interest to declare.
References
- 1.Cherian J, Rao AR, Thwaini A, et al. Medical and surgical management of priapism. Postgrad Med J 2006;82:89-94. 10.1136/pgmj.2005.037291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kheirandish P, Chinegwundoh F, Kulkarni S. Treating stuttering priapism. BJU Int 2011;108:1068-72. 10.1111/j.1464-410X.2011.10367.x [DOI] [PubMed] [Google Scholar]
- 3.Mantadakis E, Cavender JD, Rogers ZR, et al. Prevalence of priapism in children and adolescents with sickle cell anemia. J Pediatr Hematol Oncol 1999;21:518-22. 10.1097/00043426-199911000-00013 [DOI] [PubMed] [Google Scholar]
- 4.Hamre MR, Harmon EP, Kirkpatrick DV, et al. Priapism as a complication of sickle cell disease. J Urol 1991;145:1-5. 10.1016/S0022-5347(17)38229-0 [DOI] [PubMed] [Google Scholar]
- 5.Salonia A, Eardley I, Giuliano F, et al. European Association of Urology guidelines on priapism. Eur Urol 2014;65:480-9. 10.1016/j.eururo.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 6.Canguven O, Burnett AL. The effect of 5 alpha-reductase inhibitors on erectile function. J Androl 2008;29:514-23. 10.2164/jandrol.108.005025 [DOI] [PubMed] [Google Scholar]
- 7.Gur S, Kadowitz PJ, Hellstrom WJ. Effects of 5-alpha reductase inhibitors on erectile function, sexual desire and ejaculation. Expert Opin Drug Saf 2013;12:81-90. 10.1517/14740338.2013.742885 [DOI] [PubMed] [Google Scholar]
- 8.Amory JK, Wang C, Swerdloff S, et al. The effect of 5α reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J Clin Endocrinol Metab 2007;92:1659-65. 10.1210/jc.2006-2203 [DOI] [PubMed] [Google Scholar]
- 9.Barroso U, Jr, Marques TC, Novaes HF. Finasteride for recurrent priapism in children and adolescents: a report on 5 cases. Int Braz J Urol 2012;38:682-6. 10.1590/S1677-55382012000500014 [DOI] [PubMed] [Google Scholar]
- 10.Rachid-Filho D, Cavalcanti AG, Favorito LA, et al. Treatment of recurrent priapism in sickle cell anemia with finasteride: a new approach. Urology 2009;74:1054-7. 10.1016/j.urology.2009.04.071 [DOI] [PubMed] [Google Scholar]
- 11.Bramson HN, Hermann D, Batchelor KW, et al. Unique Preclinical Characteristics of GG745, A Potent Dual Inhibitor of 5AR. J Pharmacol Exp Ther 1997;282:1496-502. [PubMed] [Google Scholar]
- 12.Clark RV, Hermann DJ, Cunningham GR, et al. Marked Suppression of Dihydrotestosterone in Men with Benign Prostatic Hyperplasia by Dutasteride, a Dual 5α-Reductase Inhibitor. J Clin Endocrinol Metab 2004;89:2179-84. 10.1210/jc.2003-030330 [DOI] [PubMed] [Google Scholar]
- 13.Roehrborn CG, Marks LS, Fenter T, et al. Efficacy and safety of dutasteride in the four-year treatment of men with benign prostatic hyperplasia. Urology 2004;63:709-15. 10.1016/j.urology.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 14.Champion HC, Bivalacqua TJ, Takimoto E, et al. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci U S A 2005;102:1661-6. 10.1073/pnas.0407183102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lugg JA, Rajfer J, González-Cadavid NF. Dihydrotestosterone Is the Active Androgen in the Maintenance of Nitric Oxide-Mediated Penile Erection in the Rat. Endocrinology 1995;136:1495-501. 10.1210/endo.136.4.7534702 [DOI] [PubMed] [Google Scholar]
- 16.Schirar A, Bonnefond C, Meusnier C, et al. Androgens modulatenitric oxide synthase messenger ribonucleic acid expression in neurons of the major pelvic ganglion in the rat. Endocrinology 1997;138:3093-102. 10.1210/endo.138.8.5310 [DOI] [PubMed] [Google Scholar]
- 17.Abern MR, Levine LA. Ketoconazole and Prednisone to Prevent Recurrent Ischemic Priapism. J Urol 2009;182:1401-6. 10.1016/j.juro.2009.06.040 [DOI] [PubMed] [Google Scholar]
- 18.Traish AM, Guay AT. Are androgens critical for penile erections in humans? Examining the clinical and preclinical evidence. J Sex Med 2006;3:382-404; discussion 404-7. 10.1111/j.1743-6109.2006.00245.x [DOI] [PubMed] [Google Scholar]
- 19.Hoeh MP, Levine LA. Prevention of recurrent ischemic priapism with ketoconazole: evolution of a treatment protocol and patient outcomes. J Sex Med 2014;11:197-204. 10.1111/jsm.12359 [DOI] [PubMed] [Google Scholar]
- 20.Hoeh MP, Levine LA. Management of Recurrent Ischemic Priapism 2014: A Complex Condition with Devastating Consequences. Sex Med Rev 2015;3:24-35. 10.1002/smrj.37 [DOI] [PubMed] [Google Scholar]
- 21.Kousournas G, Muneer A, Ralph D, et al. Contemporary best practice in the evaluation and management of stuttering priapism. Ther Adv Urol 2017;9:227-38. 10.1177/1756287217717913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh PC, Madden JD, Harrod MJ, et al. Familial incomplete male pseudohermaphroditism, type 2. Decreased dihydrotestosterone formation in pseudovaginal perineoscrotal hypospadias. N Engl J Med 1974;291:944-9. 10.1056/NEJM197410312911806 [DOI] [PubMed] [Google Scholar]


