Graphical abstract
Protocol name: Hydrothermal Synthesis of LaFeO3 Nanoparticles Adsorbent: Characterization and Application of Error Functions for Adsorption of Fluoride
Keywords: Fluoride, Lanthanum ferrite nanoparticles, Langmuir, Adsorption, Isotherm
Abstract
The adsorption of fluoride from aqueous solution by lanthanum ferrite nanoparticles (LaFeO3 NPs) synthesized by the hydrothermal method has been investigated. This experimental study was conducted on a laboratory scale. The effects of various operating parameters such as pH (3–11), LaFeO3 NPs dosage (0.1–1.0 g/L), contact time (15–120 min), temperature (303–318 K), and initial concentration of fluoride (15–40 mg/L) on fluoride adsorption were studied. The results showed that under optimal conditions of fluoride concentration of 20 mg/L, pH of 5, LaFeO3 NPs dosage of 0.9 g/L, temperature of 308 K, and contact time of 60 min, maximum percentage removal of 94.75 % was obtained. The process of fluoride adsorption on LaFeO3 NPs was found to depend on the Freundlich adsorption and Koble–Corrigan isotherm models. The monolayer adsorption capacity of LaFeO3 NPs was 2.575 mg/g. The kinetic data fitted best into the pseudo-second-order model considering the values of the regression coefficients (r2) and error functions used. The thermodynamics study indicated that the adsorption process was exothermic (ΔH°< 0) and spontaneous (ΔG°< 0) in nature. It could be concluded that the synthesized LaFeO3NPs can be used as an effective adsorbent for fluoride ions removal from aqueous solutions.
Specification Table
| Subject Area: | Environmental Science |
| More specific subject area: | Adsorption |
| Protocol name: | Hydrothermal Synthesis of LaFeO3 Nanoparticles Adsorbent: Characterization and Application of Error Functions for Adsorption of Fluoride |
| Reagents/tools: | Required reagents
|
| Experimental design: | The influence of pH, contact time, initial fluoride concentration, temperature and LaFeO3 NPs dose on fluoride adsorption process. Spectral properties of LaFeO3 NPs. Adsorption kinetic, isotherm and thermodynamic parameters were also presented. |
| Trial registration: | Not applicable |
| Ethics: | Not applicable |
Value of the Protocol
|
Description of protocol
Fluoride is one of the soluble ions in water resources that originate from natural and artificial sources including wastewater discharge of different industries and fluoride glass production industry [1]. It is also a natural element among minerals, geochemical sediments, and natural water systems, which enters the food chain through drinking water or feeding on plants. When its content in water is low, it should be added to water artificially. The presence of fluoride in water is essential to prevent dental decay [2]. On the other hand, if its content exceeds the desired level, it causes dental fluorosis and skeletal fluorosis [3]. This condition causes weakening of tooth and bone structure. It also declines growth and even in severe cases, it causes paralysis and death [4,5].
Today, various methods are used to remove organic and inorganic pollutants including absorption, biosorption, and adsorption by active alumina and manganese oxide coated with alumino along with various coagulators such as alum, ferric sulfate, ferro sulfate, ferric chloride, anionic, cationic, and non-ionic organic polymers [[6], [7], [8], [9]]. Physical adsorption is an efficient and economical method. Extensive research has been conducted on the adsorption of fluoride using different adsorbents including activated carbon [8]. It has also been proven that the adsorption process is a reliable treatment solution owing to minimum investment, convenient design and operation, and insensitivity to toxic compounds [9].
Recently various rare earth materials such as lanthanum, lanthanum modified activated alumina, lanthanum oxide, lanthanum impregnated green sand, cerium, and yttrium have been used as sorbents for the removal of fluoride from water [[10], [11], [12]]. Though lanthanum has got a good affinity for fluoride, there are some difficulties related to its use as an adsorbent. Recently, the magnetic properties of lanthanum ferrite nanoparticles (LaFeO3 NPs) have been extensively studied but the magnetic study of LaFeO3 NPs is rare [10]. Ant ferromagnetic nanoparticles always show unusual magnetic properties due to their finite-size effects, surface anisotropy effects, interface effects and shape anisotropy effect [[10], [11], [12]]. The nano-size of LaFeO3 NPs system has been majorly investigated as an alternative. Various types of LaFeO3 NPs can be synthesized by many methods such as sol-gel, co-precipitation, bull milling, sonochemical, and hydrothermal [10]. The hydrothermal method is one of the most powerful and widely used methods for the production of nanostructures; this has attracted a great deal of attention due to its simplicity and cost [13].
The main purpose of this study is to synthesize LaFeO3 NPs and investigate its effectiveness on the removal of fluoride from its aqueous solution. The impact of various operating factors such as contact time, LaFeO3 NPs dosage, pH, temperature and initial fluoride concentration on the fluoride adsorption process was studied to ascertain their optimum conditions. The adsorption kinetics, isotherm, and thermodynamics of the fluoride adsorption process on LaFeO3 NPs will also be studied. Error functions were also employed to compare the fit of adsorption isotherm and kinetic models in order to limit error between the predicted and experimental values.
Materials and methods
Preparation of LaFeO3 NPs
Lithium nanostructure was used with poly vinyl pyrrolidone (PVP) coating agent, and was then dissolved in distilled water using hydrothermal method with equal ratios of iron salt (Fe(NO3).9H2O) and lanthanum salt (La(NO3)3.6H2O) (that is, 0.2 g each was dissolved in 20 mL of distilled water and added to each other).
In another container, 0.5 g of PVP which had been dissolved in 40 mL of distilled water (at a temperature of 25 °C) was added to the reaction container. After vigorous stirring for 30 rpm, NaOH alkaline agent was added to the reaction container in order to raise the pH to 11. Next, the intended solution was transferred to an autoclave and placed inside an oven for 24 h at 200 ⁰C. Thereafter, the obtained solution was washed several times with distilled water and ethanol and then dried in an oven for 30 min at 343 K.
Characterization of nanometer-sized LaFeO3 NPs
X-ray diffraction (XRD) patterns on the LaFeO3 NPs were taken by means of a Philips diffract meter model PW1800 (The Netherlands). The X-ray source was Cukα with 1.541 nm wavelength. Scanning electron microscopy (Mira 3-XMU instrument capable of 700,000 x magnifications) was used to study the morphology of the LaFeO3 NPs. Fourier-transform infrared spectroscopy (FT-IR) analysis of LaFeO3 NPs was done using a JASCO 640 plus machine (4000−400 cm−1) at room temperature to determine the functional groups presently involved in the fluoride adsorption process.
Batch adsorption experiments
The effects of different parameters such as pH (3, 5, 7, 9 and 11), contact time (15, 30, 60, 90, and 120 min), temperature (303, 308, and 318 K), initial fluoride concentration (15, 20, 25, 30 and 40 mg/L) and LaFeO3 NPs dosage (0.1, 0.5, 0.7, 0.9 and 1 g/L) on the fluoride adsorption process were studied. A specified amount of adsorbent (LaFeO3 NPs) was added to Erlenmeyer flasks containing 100 mL of the solutions to be treated having different concentrations of fluoride. The pH of the solution was adjusted by adding 0.1 N HCl or 0.1 N NaOH. The flask with its contents was stirred for a specified time at 150 rpm. The resulting solution was centrifuged and the supernatant was analyzed for the residual fluoride concentration. The initial and final (or residual) fluoride concentrations in the solutions were determined using a UV–vis spectrophotometer (Shimadzu Model: CE-1021-UK, Japan) at a wavelength of maximum absorbance (λmax) of 570 nm [14]. The pH was measured using a MIT65 pH meter. The removal efficiency (%R) was calculated based on the following formula [15,16]:
| (1) |
The amount of fluoride adsorbed on LaFeO3 NPs, qe(mg/g) was calculated based on the following formula [17]:
| (2) |
Where C0 and Ce are the initial fluoride concentration (mg/L) and equilibrium liquid phase concentration of fluoride (mg/L) respectively; Cf is the final fluoride concentration (mg/L), V is the volume of the treated fluoride solution (L) and M is the amount of LaFeO3 NPs used (g).
Results and discussion
SEM, XRD and FTIR analysis on the synthesized LaFeO3 NPs
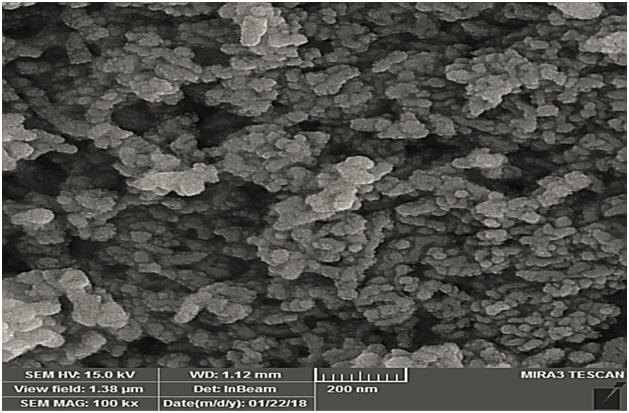
The scanning electron microscopy (SEM) image of LaFeO3 NPs is shown in Fig. 1. The LaFeO3 NPs appears lamellar in arrangement. The surface area of an adsorbent determines its adsorption capability [18]. High porosity was observed on the LaFeO3 NPs which indicates that there will be a high level of contact with the fluoride ions [20].The X-ray diffraction (XRD) patterns of LaFeO3 NPs is shown in Fig. 2.
Fig. 1.
SEM image of synthesized LaFeO3 NPs prepared by hydrothermal method.
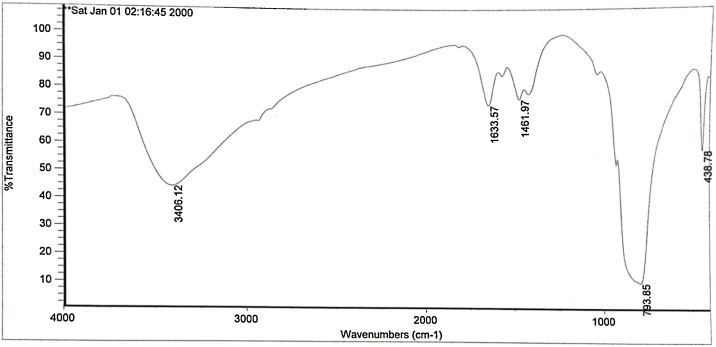
Fig. 2.
FTIR spectra of the LaFeO3 NPs prepared by the hydrothermal method.
The FTIR analysis on theLaFeO3 NPs (Fig. 2) indicates the existence of C—H bend of alkenes (793.85 cm−1), and C—H bend of alkanes (1461.97 cm-1). The peak 1633.57 cm-1 shows the presence of C—C stretch (in-ring) of aromatics. The presence of O—H stretch, H–bonded of alcohols, phenols (3406.12 cm-1), which are also strong and broad bands can be observed. The O—H bands are very important sites for adsorption [21]. The hydroxyl group effect is more felt due to the hydrogen bonding with other hydroxyl bonds since they do not exist in isolation establishing a stable structure [21,22].
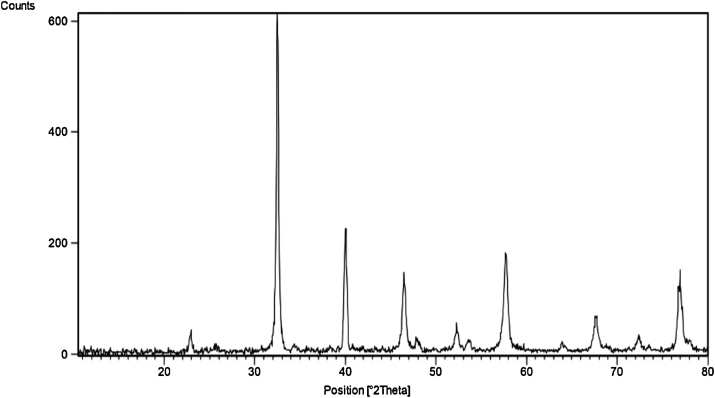
The XRD result shows that the LaFeO3 NPs owns a crystalline structure which improves the process of adsorption by means of physical adsorption [19,20]. Maximum peak of around 2θ = 32.5° (with very high intensity) was also observed on the XRD image (Fig. 3). The average crystallite size (D) of LaFeO3 NPs nanoparticles was calculated by the Scherer formula (Dh,k,l = 0.9λ/βh,k,l cosθ, where λ is the wavelength (1.542 Å), β is the full width at half maximum (FWHM) of the line, and θ is the diffraction angle) [23,24]. The average diameter of the LaFeO3 NPs adsorbent (D) was calculated to be 35 nm.
Fig. 3.
XRD patterns of the LaFeO3 NPs prepared by the hydrothermal method.
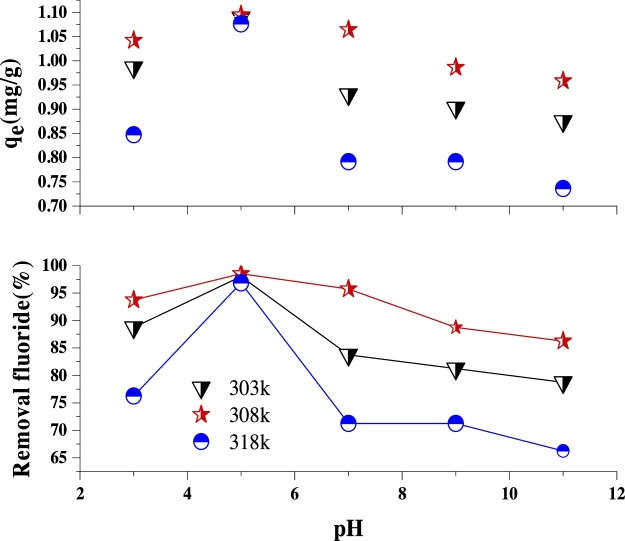
The effect of pH and temperature
The parameter, pH directly influences the electrostatic interaction between compounds in adsorption processes [25]. To obtain optimal pH value, experiments were carried out by varying the initial pH from 3 to 11, at the constant fluoride concentration of 20 mg/L, LaFeO3 NPs dosage of 1 g/L, and contact time of 15 min at different temperatures of 303, 308, and 318 K. The influence of pH with temperature on the percentage removal of fluoride and the amount of fluoride adsorbed (qe) on LaFeO3 NPs surface are shown in Fig. 4. Fluoride reduction was maximum at the temperature of 308 K, which is accepted as the optimum temperature. The temperature increase from 308 to 318 K also allowed the fluorine to desorb to the solution due to damage to the active sites in the adsorbent [26]. Also, it is clear from Fig. 4 that the removal percentage of fluoride increased from 93.75%–98.525 % as the pH was increased from 3 to 5 at the temperature of 308 K but decreased as the pH was increased to 11. Many researchers have reported that the adsorption process is affected by the cationic and anionic forms of the solution due to competition for adsorption among the H+ and OH– ions with the adsorbate [27]. In this study, the decrease in the removal efficiency of fluoride as the pH increased may be attributed to electrostatic repulsion [28] between the positively charged LaFeO3 NPs and the cationic fluoride. The adsorption of fluoride was more favorable in the acidic environment due to the presence of H+ on the adsorbent [29]. The increased amount of H+ and reduction of OH– as well as the increase of positive ion can be the reason for the reduction in fluoride removal efficiency on the adsorbent surface [30]. This is also due to the competition of the fluoride ions with excess OH– ions for the adsorption sites at higher adsorption pH [29,30].
Fig. 4.
Effect of pH on fluoride adsorption ontoLaFeO3NPs (LaFeO3NPs dosage: 1 g/L, initial fluoride concentration: 20 mg/L, and contact time: 15 min).
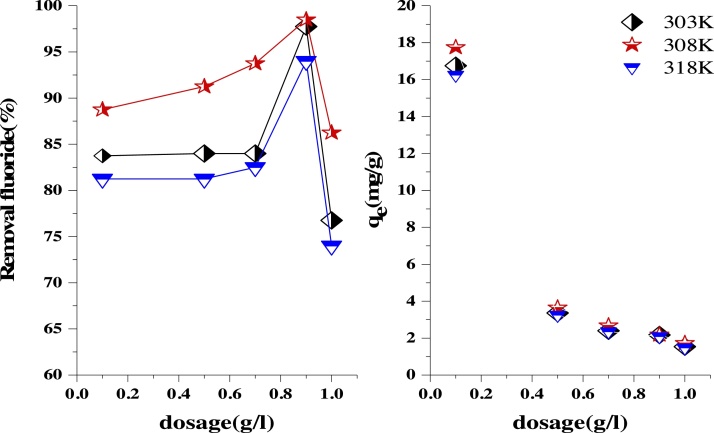
The effect of adsorbent dosage
As seen in Fig. 5, the removal of the fluoride increased with increasing the amount of adsorbent (LaFeO3 NPs) from 0.1 to 0.9 g/L at different temperatures (303, 308 and 318 K). Maximum fluoride uptake of 98.5 % was observed at an adsorbent dosage of 0.9 g/L and temperature of 308 K. This implies that increasing LaFeO3 NPs dose increased the number of active sites available for the adsorption of fluoride. Therefore, the studied adsorbent has a high adsorptive potential, which at very low adsorbent values has a very high uptake of fluoride. With increasing the adsorbent dosages above the optimum (0.9 g/L), the fluoride removal was decreased, which is due to the accumulation of adsorbent particles and the development of electric repulsive force between the adsorbent particles. It can be pointed out that all active sites of the adsorbent were not available to the adsorbate, with this phenomenon being observed more in the batch adsorption process [31].
Fig. 5.
Effect of adsorbent dosage on fluoride adsorption ontoLaFeO3 NPs (pH: 5, initial fluoride concentration: 20 mg/L, and contact time: 15 min).
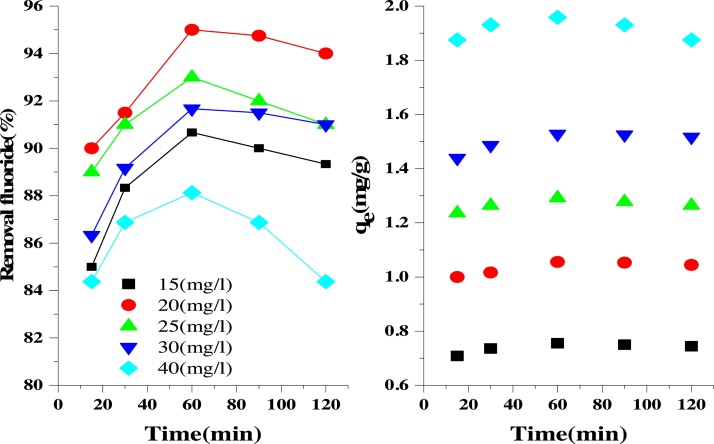
The effect of fluoride concentration and contact time
It is important to note that the adsorbate concentration plays a significant role in the removal of pollutants from aqueous solutions and the interaction between the adsorbent and adsorbate species. The effect of concentration on the fluoride adsorption by the LaFeO3 NPs was investigated at different initial fluoride concentrations (15, 20, 25, 30, and 40 mg/L) at pH of 5, contact time of 15 min and LaFeO3 NPs dosage of 0.9 g/L at different temperatures (Fig. 6). The amount of fluoride adsorbed on LaFeO3 NPs (qe) was increased with increasing fluoride concentration. Also, the percentage of fluoride adsorbed was increased as the initial concentration of fluoride was increased from 15 to 20 mg/L but decreased when the fluoride concentration was increased further at different times of contact. This decrease in efficiency of fluoride removal may be as a result of the over-saturation of the active sites of the adsorbent by the adsorbate [32].
Fig. 6.
Effect of fluoride concentration and contact time on fluoride adsorption onto LaFeO3 NPs (pH: 5, LaFeO3 NPs dosage: 0.9 g/L, and temperature =308 K, stirring speed = 150 rpm).
The effect of contact time (15, 30, 60, 90 and 120 min) on the removal of fluoride was studied at pH of 5, LaFeO3 NPs dosage of 0.9 g/L, and fluoride concentration of 25 mg/L at different fluoride concentrations (Fig. 6). From Fig. 6 it can be seen that the removal of fluoride increased as contact time increases from 10 to 60 min. Maximum removal of fluoride was achieved in the first 60 min (94.75 %) at the concentration of 20 mg/l. The adsorption of fluoride in the initial minutes was high, including the adsorption rate (Fig. 6) because of reduced fluoride concentration and reduction of the active sites present on the adsorbent surface [33]. The removal efficiency decreased after 60 min because the adsorption sites were occupied [34].
Sorption kinetics fitting
It is important to emphasize that the optimal contact time is determined based on the adsorption kinetics tests. Kinetic studies are done to observe the mechanism controlling an adsorption process [26]. Kinetic models of adsorption including pseudo-first-order, pseudo-second-order, intraparticle diffusion, fractional and zero-order models were used to test the kinetic data. The equations of kinetic models with the description of the kinetic parameters are stated in Table 1. The kinetic parameters were obtained from the plots of the kinetic models at the optimum conditions of pH 5 and nanoparticles dose of 0.9 g/L. The agreement between the predicted kinetic model values and the experimental data was confirmed by the regression coefficients (r2).
Table 1.
Kinetic models employed to describe the fluoride adsorption by LaFeO3 NPs with their respective equations and parameters description [28,29].
| Kinetic model | Equation | Parameters description |
|---|---|---|
| Pseudo-second-order | k2p = rate constants of the pseudo-second order (g/mg min); qe= adsorbate amounts at equilibrium (mg/g); qt = amount of adsorbate removed at time t (mg/g). | |
| Pseudo-first-order | k1p= rate constants of the pseudo- first-order (min−1); qe= adsorbate amount at equilibrium (mg/g); qt = amount of adsorbate removed at time t (mg/g) | |
| Intra-particle diffusion | qt = amount of adsorbate adsorbed at equilibrium (mg/g); kp = intraparticle diffusion rate constant (mg/L min−0.5); C = the intercept which give information about the thickness of the boundary layer | |
| Fractional power | k= constant; v= rate constant | |
| Zero-order | qe = adsorbate amounts at equilibrium (mg/g); k0 =constant |
The nonlinear forms of pseudo-first-order, pseudo-second-order, intraparticle diffusion, fractional and zero-order models were used to test the kinetic data. In order to evaluate the validity of the adsorption mathematical kinetic models with the experimental results, a number of error functions are available in the literature. The use of only the regression coefficient (r2) for isotherm and kinetic data analysis is not enough, because the experimental results may have high r2 values. Therefore, it is necessary to diagnose the result of regression for residue analysis. The applicability of the kinetic model to describe the adsorption process, apart from the regression coefficient (r2), was further validated by the normalized standard deviation (NSD), average relative error (ARE) and standard deviation which are defined as Eqs. (3), (4), (5), respectively:
| (3) |
| (4) |
| (5) |
Where N is the number of performed experiments, P is the number of parameters of the fitted model, and r2 is the coefficient of determination; qtexp and qtcal are the experimental and calculated amounts of fluoride adsorbed on LaFeO3 NPs at time t (mg/g). The model with the highest values of r2 and the lowest values of SD best represents the process. The smaller NSD and ARE values indicate a more accurate estimation of qt values [20,25].
The nonlinear adsorption kinetics results obtained are presented in Table 2. It is observed that the pseudo-second-order kinetic model best described the kinetic experimental data with the value of r2 closer to unity (0.8577). The pseudo-second-order possesses lower values of NSD (0.8873), ARE (0.7120), and SD (0.0106) when compared with the other kinetic models. This means that the fluoride adsorption onto LaFeO3 NPs is a chemical type of adsorption [35]. This also indicates that the calculated values of qt (qtcal) obtained from the pseudo-second-order model extremely correspond with the experimental values of qt (qtexp).
Table 2.
Nonlinear kinetic parameters for adsorption of fluoride onto LaFeO3 NPs at optimal condition (pH: 5, nanoparticles dose: 0.9 g/L, temperature =308 K).
| Model | r2 | NSD | ARE(%) | SD | |
|---|---|---|---|---|---|
| Pseudo-second-order | 0.8577 | 0.8873 | 0.7120 | 0.0106 | |
| 0.9922 | |||||
| 1.06 | |||||
| Pseudo-first-order | 0.6422 | 1.4232 | 0.9592 | 0.0169 | |
| 1.043 | |||||
| 0.2088 | |||||
| Intra-particle diffusion | 0.7108 | 1.2542 | 0.9498 | 0.0152 | |
| 0.007144 | |||||
| 0.9803 | |||||
| Fractional power | 0.7991 | 1.0431 | 0.7734 | 0.0126 | |
| 0.9377 | |||||
| 0.02502 | |||||
| Zero-order | 0.5977 | 1.4849 | 1.1560 | 0.0179 | |
| −0.0004393 | |||||
| 1.006 |
Equilibrium isotherms and fit error evaluation
In different adsorption investigations, the study of the adsorption of pollutants on the surface of adsorbents, determining the adsorption capacity (qm) and adsorption isotherm models that best fit the experimental data are of great importance to many researchers. The way a pollutant is adsorbed on an adsorbent can be interpreted through the study of adsorption isotherms. Isotherms can represent the relationship between the pollutant concentration present in the solution and the amount of pollutant adsorbed by the solid phase when both phases are at equilibrium. The equations of isotherm models with the description of their parameters are stated in Table 3. The correlation coefficient (r2) is used to judge whether experimental data follow isotherm models [36]. In addition to r2, the parameters of average relative error (ARE), Marquardt’s percent standard deviation (MPSD) and Hybrid error function (HYBRID), root mean squared error (RMSE), and normalized standard deviation (Δq(%)) were also evaluated, which can be described as Eqs. (4), (6), (7), (8), (9), respectively:
| (6) |
| (7) |
| (8) |
| (9) |
Where qeiexp is the observation from the batch experiment i, qeical is the estimate from the isotherm for the corresponding qeiexp, Nis the number of observations in the experimental isotherm and p is the number of parameters in the regression model; qc is the value that is calculated from model fit and qe is calculated from test elements. The smaller MPSD and HYBRID values indicate a more accurate estimation of qe value [20]. MPSD and HYBRID functions were used in addition to r2 because the number of parameters in the regression model (that is, p parameter) is effective in them.
Table 3.
Isotherm models employed to describe the fluoride adsorption by LaFeO3 NPs with their respective equations and parameters description [[37], [38], [39]].
| Isotherm model | Equation | Parameters description |
|---|---|---|
| Langmuir | KL = Langmuir constant (L/mg); qe = amount of adsorbate adsorbed (mg/g); qm = maximum/monolayer adsorption capacity (mg/g); Ce = equilibrium concentration of adsorbate in solution (mg/L) | |
| Freundlich | KF = Freundlich constant; qe = amount of adsorbate adsorbed (mg/g); Ce = equilibrium concentration of adsorbate in solution (mg/L), n = intensity of adsorption | |
| Temkin | Ce = equilibrium concentration of adsorbate in solution (mg/L) ; qe = amount of adsorbate adsorbed (mg/g); B1 = heat of sorption; AT= equilibrium binding constant | |
| Koble–Corrigan | Ce = equilibrium concentration of adsorbate in solution (mg/L); qe = amount of adsorbate adsorbed (mg/g); BKC, AKCandp = Koble–Corrigan isotherm constants | |
| Redlich–Peterson | Ce = equilibrium concentration of adsorbate in solution (mg/L); qe = amount of adsorbate adsorbed (mg/g); ARP=Redlich–Peterson isotherm constant (L/g);BRP= constant (L/mg), and g = exponent that lies between 0 and 1. | |
| Dubinin–Radushkevich |
|
Ce = equilibrium concentration of adsorbate in solution (mg/L); qD = theoretical saturation capacity (mg/g) ; qe = amount of adsorbate adsorbed (mg/g); D = constant related to mean free energy of adsorption per mole of the adsorbate (mol2/J2); R= universal gas constant (8.314 J/mol/K) ; ε = polanyi potential |
The isotherm plots for fluoride adsorption by LaFeO3 NPs at the optimum conditions of pH 5, LaFeO3 NPs dose of 0.9 g/L, and temperature of 308 K is shown in Fig. 7. Considering the r2 values obtained for the theoretical models evaluated (Table 4), it can be observed that the six isotherm models (Langmuir, Freundlich, Temkin, Koble–Corrigan, Redlich–Peterson and Dubinin–Radushkevich) present firm adherence to the experimental data. This shows the good agreement between the calculated qe and the experimental qe values for all isotherms. By evaluating the values of all the error functions applied to the adjusted models, the Freundlich and Koble–Corrigan models were the most suitable to describe the observed phenomenon. Its fit into the Freundlich model suggests a heterogeneous and multilayer adsorption of the fluoride on the LaFeO3 NPs surface. Since the Freundlich model describes a chemical adsorption process, it supports the kinetic approach which denoted a chemical behavior of the adsorption of fluoride on LaFeO3 NPs. The monolayer adsorption capacity of LaFeO3 NPs (qm) was obtained as 2.575 mg/g. An adsorption intensity (n) value of 2.488, which is within 1–10 (1 <n< 10) obtained for the fluoride adsorption proposes that the adsorption process on LaFeO3 NPs is favorable [20].
Fig. 7.
Isotherm plots for fluoride adsorption by LaFeO3 NPs (Adsorption conditions: pH = 5, LaFeO3 NPs dose = 0.9 g/L, stirring speed = 150 rpm, temperature =308 K).
Table 4.
Isotherms parameters provided by isotherm models, with the error functions evaluated for sorption of fluoride by LaFeO3 NPs.
| Model | r2 | MSPD | HYBRID | RMSE | ARE (%) | ||
|---|---|---|---|---|---|---|---|
| Langmuir | 0.9788 | 5.7628 | 0.3988 | 2.48E-03 | 3.6762 | 22.1396 | |
| 2.575 | |||||||
| 0.6225 | |||||||
| Freundlich | 0.9985 | 1.3841 | 0.0250 | 1.66E-04 | 0.8230 | 10.4752 | |
| 1.048 | |||||||
| n | 2.488 | ||||||
| Temkin | 0.9870 | 4.2127 | 0.2222 | 1.44E-03 | 2.7356 | 19.0982 | |
| 0.5859 | |||||||
| 5.644 | |||||||
| Koble–Corrigan | 0.9985 | 1.9102 | 0.0487 | 1.64E-04 | 0.8080 | 10.3792 | |
| 1.02 | |||||||
| 0.3862 | |||||||
| −0.02757 | |||||||
| Redlich–Peterson | 0.9976 | 2.7400 | 0.0896 | 2.71E-04 | 1.0819 | 12.0104 | |
| 6.216 | |||||||
| g | 0.666 | ||||||
| 4.998 | |||||||
| Dubinin–Radushkevich | 0.8724 | 12.1894 | 2.0137 | 1.44E-02 | 8.4500 | 33.5659 | |
| 1.921 | |||||||
| 2.237E-07 |
Thermodynamic studies
Temperature has a great impact on the adsorption process, so the thermodynamic study. The thermodynamic parameters including the standard Gibbs free energy (ΔG°), enthalpy change (ΔH°), and entropy change (ΔS°) are useful in defining whether the sorption reaction is endothermic or exothermic, and spontaneity of the adsorption process. The thermodynamic parameters (ΔG°, ΔH°, and ΔS°) for adsorption of fluoride onto LaFeO3 NPs were calculated (Table 5) using the following equations [20]:
| (10) |
| (11) |
Where, R is the universal gas constant (8.314 J/mol/K) and T is the absolute temperature in K.
Table 5.
Thermodynamic parameters for the adsorption of fluoride using LaFeO3 NPs.
| Temperature (K) | C0 (mg/L) | ΔS° (kJ/mol.K) | ΔH° (kJ/mol) | ΔG° (kJ/mol) |
|---|---|---|---|---|
| 298 | −3.94 | |||
| 308 | 20 | −0.0115 | −0.5057 | −4.06 |
| 318 | −4.17 |
The thermodynamic parameter, Gibb’s free energy change (ΔG°) is calculated using Ka obtained from the Langmuir isotherm. The values of ΔH° and ΔS° were evaluated from the intercept and slope of the regression plot of ΔG° versus T (Fig. 8).
Fig. 8.
The plot of Gibbs free energy change, ΔG°, versus temperature, T.
All the values of were negative; this shows that the fluoride adsorption process by LaFeO3 NPs was spontaneous (ΔG°< 0) and feasible [40]. The decreased amount of with the increase in temperature indicates that the increase in temperature resulted in an increase in spontaneity. The negative value of adsorption reaction on LaFeO3 NPs (−0.5057 kJ/mol) indicated that the process was exothermic (ΔH°< 0) [41]. According to Le Chatelier's principle, increasing the temperature reduced the reaction rate. Entropy change (ΔS° = -0.0115 kJ/mol.K) of fluoride adsorption by LaFeO3 NPs is negative, suggesting that the degree of freedom at solid-solution level declines during the adsorption [30]. The negative value of ΔS° may be caused by the decrease in the efficiency of the reaction with higher temperatures [42,43].
Comparison of LaFeO3 NPs with other adsorbent materials on fluoride removal
The removal of fluoride on LaFeO3 NPs was compared with other adsorbent materials employed by several authors in terms of percentage removal efficiency (Table 6). The fluoride removal efficiency of 94.75 % obtained using LaFeO3 NPs indicates that it can be applied for fluoride removal from its aqueous solution. Generally, the results obtained by the authors shown in Table 6 show that the different adsorbents can be harnessed for the removal of fluoride via the adsorption process.
Table 6.
Comparison of LaFeO3 NPs with other adsorbents for fluoride reduction.
| Adsorbent material | Maximum removal (%) | Conditions | Reference |
|---|---|---|---|
| Nickel oxide nanoparticles | 98.75 | pH = 5; Adsorbent dosage =0.02 g; Initial concentration = 20 mg/L; Time =60 min; Volume of fluoride solution, V = 100 mL; Temperature =298 K ; Speed = 150 rpm |
[44] |
| Synthesized P/γ-Fe2O3 nanoparticles | 99 | pH = 7; Adsorbent dosage =0.02 g/L; Initial concentration =25 mg/L; Time =30 min; V = 1 L; Speed = 150 rpm |
[14] |
| Chitosan | 87 | pH = 7; Adsorbent dosage =5 g/L; Initial concentration =5 mg/L; Time = 180 min |
[45] |
| Modified Turkish zeolite with quaternary ammonium | 85 | pH = 5; Adsorbent dose = 20 mg/L; Contact time =60 min; Temperature =293 K; Initial fluoride concentration = 10 mg/L V = 100 mL; Stirring speed = 200 rpm |
[26] |
| Peanut husk | 82.3 | pH = 3; Adsorbent dosage =6 g/L; Initial concentration = 10 mg/L; Time =80 min; V = 100 mL; Temperature = 23 ± 2 °C |
[46] |
| Lanthanum ferrite nanoparticles (LaFeO3 NPs) | 94.75 | pH = 5; LaFeO3 NPs dosage = 0.9 g/L; Fluoride concentration = 20 mg/L; Time =60 min; V = 100 mL; Temperature =308 K; Speed = 150 rpm |
This study |
Conclusion
The removal of fluoride on lanthanum ferrite nanoparticles (LaFeO3 NPs) was found to be dependent on the initial pH, temperature, dosage of LaFeO3 NPs, contact time, and initial fluoride concentration. Under optimal conditions of fluoride concentration of 20 mg/L, pH of 5, LaFeO3 NPs dosage of 0.9 g/L, temperature of 308 K, and contact time of 60 min, maximum percentage removal of 94.75 % was obtained. Adsorption kinetics, isotherm, and thermodynamics were studied for fluoride ions removal on LaFeO3 NPs. The monolayer adsorption capacity of LaFeO3 NPs was 2.575 mg/g. The adsorption process fitted well into the Freundlich, Koble–Corrigan and pseudo-second-order kinetic models considering the values of the regression coefficients (r2) and error functions used. The fluoride adsorption on LaFeO3 NPs was found to be favorable, exothermic and spontaneous in nature. Its spontaneity was increased with temperature. From the study, it can be concluded that the LaFeO3 NPs prepared by the hydrothermal method can be used for the effective reduction of fluoride concentration in aqueous environments. Since simulated fluoride effluent was used in the present study, further studies can be carried out on real fluoride-containing wastewater.
Funding sources
The authors thank the Research Assistance of Zabol University of Medical Sciences (No. IR.ZBMU. REC.1396.330) for financial and spiritual.
Declaration of Competing Interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Mohammad Mesbah, Email: m_mesbah@alum.sharif.edu.
Shahin Ahmadi, Email: Sh_ahmadi@zbmu.ac.ir.
Chinenye Adaobi Igwegbe, Email: ca.igwegbe@unizik.edu.ng.
References
- 1.Emamjomeh M.M., Sivakumar M., Varyani A.S. Analysis and the understanding of fluoride removal mechanisms by an electrocoagulation/flotation (ECF) process. Desalination. 2011;275:102–106. doi: 10.1016/j.desal.2011.02.032. [DOI] [Google Scholar]
- 2.Mohapatra M., Anand S., Mishra B.K., Giles D.E., Singh P. Review of fluoride removal from drinking water. J. Environ. Manage. 2009;91:67–77. doi: 10.1016/j.jenvman.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Mohammadnia M., Naghizadeh A. Surveying of kinetics, thermodynamic, and isotherm processes of fluoride removal from aqueous solutions using graphene oxide nano particles. J. Birjand Univ. Med. Sci. 2016;23:29–43. [Google Scholar]
- 4.Takdastan A., Tabar S.E., Islam A., Bazafkan M.H., Naisi A.K. The effect of the electrode in fluoride removal from drinking water by electro coagulation process. International Conference on Chemical, Environmental and Biological Sciences. 2015:39–44. doi: 10.15242/IICBE.C0315073. [DOI] [Google Scholar]
- 5.Tripathy S.S., Bersillon J.L., Gopal K. Removal of fluoride from drinking water by adsorption onto alum-impregnated activated alumina. Sep. Purif. Techn. 2006;50:310–317. doi: 10.1016/j.seppur.2005.11.036. [DOI] [Google Scholar]
- 6.Emamjomeh M.M., Sivakumar M. Fluoride removal by a continuous flow electrocoagulation reactor. J. Environ. Manage. 2009;90:1204–1212. doi: 10.1016/j.jenvman.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J., Zhao H., Ni J. Fluoride distribution in electrocoagulation defluoridation process. Sep. Purif. Technol. 2007;56:184–191. doi: 10.1016/j.seppur.2007.01.030. [DOI] [Google Scholar]
- 8.Jamhour R.M. New inorganic ion-exchange material for the selective removal of fluoride from potable water using ion-selective electrode. Am. J. Environ. Sci. 2005;1(1):1–4. doi: 10.3844/ajessp.2005.1.4. [DOI] [Google Scholar]
- 9.Samadi M.T., Nourozi R., Azizian S., Dadban Shahamat Y., Zarabi M. Servey impact of activated alumina in fluoride concentration present in water and appointment adsorption isotherm and kinetics. Iranian J. Health Environ. 2009;2(3):224–231. [Google Scholar]
- 10.Phokha S., Pinitsoontorn S., Maensiri S., Rujirawat S. Structure, optical and magnetic properties of LaFeO3 nanoparticles prepared by polymerized complex method. J. Solgel Sci. Technol. 2014;71(2):333–341. doi: 10.1007/s10971-014-3383-8. [DOI] [Google Scholar]
- 11.Rao C.N., Karthikeyan J. Removal of fluoride from water by adsorption onto lanthanum oxide. Water Air Soil Pollut. 2012;223(3):1101–1114. doi: 10.1007/s11270-011-0928-0. [DOI] [Google Scholar]
- 12.Peng K., Fu L., Yang H., Ouyang J. Perovskite LaFeO3/montmorillonite nanocomposites: synthesis, interface characteristics and enhanced photocatalytic activity. Sci. Rep. 2016;6:19723. doi: 10.1038/srep19723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng W.J., Liu R.H., Peng D.K., Meng G.Y. Hydrothermal synthesis of LaFeO3 under carbonate-containing medium. Mater. Lett. 2000;43:19–22. doi: 10.1016/S0167-577X(99)00223-2. [DOI] [Google Scholar]
- 14.Ahmadi S., Rahdar S., Igwegbe C.A., Rahdar A., Shafighi N., Sadeghfar F. Data on the removal of fluoride from aqueous solutions using synthesized P/γ-Fe2O3 nanoparticles: a novel adsorbent. MethodsX. 2019;6:98–106. doi: 10.1016/j.mex.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samadi M.T., Kashitarash Esfahani Z., Ahangari F., Ahmadi S., Jafari J. Nickel removal from aqueous environments using carbon nanotubes. Int. J. Water Wastewater Treat. 2013;24:38–44. [Google Scholar]
- 16.Ahmadi S., Mostafapour F.K. Treatment of textile wastewater using a combined coagulation and DAF processes, Iran, 2016. Arch. Hygiene Sci. 2017;6(3):229–234. doi: 10.29252/ArchHygSci.6.3.229. [DOI] [Google Scholar]
- 17.Ahmadi S., Kord Mostafapour F. Tea waste as a low cost adsorbent for the removal of COD from landfill leachate: kinetic study. J. Sci. Eng. Res. 2017;4:103–108. [Google Scholar]
- 18.Dehghani M.H., Faraji M., Mohammadi A., Kamani H. Optimization of fluoride adsorption onto natural and modified pumice using response surface methodology: isotherm, kinetic and thermodynamic studies. Korean J. Chem. Eng. 2017;34:454–462. doi: 10.1007/s11814-016-0274-4. [DOI] [Google Scholar]
- 19.Rahdar A., Ahmadi S., Fu J., Rahdar Somayeh. Iron oxide nanoparticle preparation and its use for the removal of fluoride from aqueous solution: application of isotherm, kinetic, and thermodynamics. Desal. Water Treat. 2019;137:174–182. doi: 10.5004/dwt.2019.23350. [DOI] [Google Scholar]
- 20.Ahmadi S., Rahdar A., Rahdar S., Igwegbe C.A. Removal of Remazol Black B from aqueous solution using P-γ-Fe2O3 nanoparticles: synthesis, physical characterization, isotherm, kinetic and thermodynamic studies. Desal. Water Treat. 2019;152:401–410. doi: 10.5004/dwt.2019.23978. [DOI] [Google Scholar]
- 21.Okeola O.F., Odebunmi E.O., Ameen O.M. Comparison of sorption capacity and surface area of activated carbon prepared from Jatropha curcas fruit pericarp and seed coat. Bull. Chem. Soc. Ethiop. 2012;2:171–180. doi: 10.4314/bcse.v26i2.2. [DOI] [Google Scholar]
- 22.Coates J. In: A Practical Approach. Encyclopedia of Analytical Chemistry. Meyers R.A., editor. John Wiley & Sons Ltd; Chichester: 2010. Interpretation of infrared spectra; pp. 10815–10837. [Google Scholar]
- 23.Rahdar S., Rahdar A., Igwegbe C.A., Moghaddam F., Ahmadi S. Synthesis and physical characterization of nickel oxide nanoparticles and its application study in the removal of ciprofloxacin from contaminated water by adsorption: equilibrium and kinetic studies. Desalin. Water Treat. 2019;141:386–393. [Google Scholar]
- 24.Hakami T.M., Davarpanah A.M., Rahdar A., Barrett S.D. Structural and magnetic study and cytotoxicity evaluation of tetra-metallic nanoparticles of Co0.5Ni0.5CrxFe2-xO4 prepared by co-precipitation. J. Mole. Structure. 2018;1165:344–348. [Google Scholar]
- 25.Ahmadi S., Igwegbe C.A., Radhar S., Asadi Z. The survey of application of the linear and non-linear kinetic models for the adsorption of nickel (II) by modified Multi-Walled Carbon Nanotubes. Appl. Water Sci. 2019;9(98) doi: 10.1007/s13201-019-0978-9. [DOI] [Google Scholar]
- 26.Aloulou H., Ghorbel A., Aloulou W., Amar R.B., Khemakhem S. Removal of fluoride ions (F−) from aqueous solutions using modified Turkish zeolite with quaternary ammonium. Environ. Technol. 2019;25:1–13. doi: 10.1080/09593330.2019.1668863. [DOI] [PubMed] [Google Scholar]
- 27.Ahmadi S., Igwegbe C.A. Adsorptive removal of phenol and aniline by modified bentonite: adsorption isotherm and kinetics study. Appl. Water Sci. 2018;8(6):170. doi: 10.1007/s13201-018-0826-3. [DOI] [Google Scholar]
- 28.Ji L.G., Chen W., Duan L., Zhu D. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: a comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 2009;43:2322–2327. doi: 10.1021/es803268b. [DOI] [PubMed] [Google Scholar]
- 29.Rahdar S., Ahmadi Sh. Removal of phenol and aniline from aqueous solutions by using adsorption on to Pistacia terebinthus: study of adsorption isotherm and kinetics. J. Health Res. Commun. 2017;2(4):35–45. [Google Scholar]
- 30.Ahmadi S., Bazrafshan E., Kord Mostafapoor F. Treatment of landfill leachate using a combined Coagulation and modify bentonite adsorption processes. J. Sci. Eng. Res. 2017;4(2):58–64. [Google Scholar]
- 31.Ahmadi S., Kord Mostafapour F. Adsorptive removal of aniline from aqueous solutions by Pistacia atlantica (Baneh) shells: isotherm and kinetic studies. J. Sci. Technol. Environ. Inform. 2017;5(1) doi: 10.18801/jstei.050117.35. 327-325. [DOI] [Google Scholar]
- 32.Saif M.M., Kumar N.S., Prasad M.N. Binding of cadmium to Strychnospotatorum seed proteins in aqueous solution: adsorption kinetics and relevance to water purification. Colloids Surf. B Biointerfaces. 2012;94:73–79. doi: 10.1016/j.colsurfb.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 33.Olsen S.R., Watanabe F.S. A method to determine a phosphorus adsorption maximum of soils as measured by the Langmuir isotherm 1. Soil Sci. Soc. Am. J. 1957;21(2):144–149. doi: 10.2136/sssaj1957.03615995002100020004x. [DOI] [Google Scholar]
- 34.Reed B.E., Matsumoto M.R. Modeling cadmium adsorption by activated carbon using the Langmuir and Freundlich isotherm expressions. Sep. Sci. Technol. 1993;28(13-14):2179–2195. doi: 10.1080/01496399308016742. [DOI] [Google Scholar]
- 35.Igwegbe C.A., Onyechi P.C., Onukwuli O.D. Kinetic, isotherm and thermodynamic modelling on the adsorptive removal of malachite green on Dacryodes edulis seeds. J. Sci. Eng. Res. 2015;2:23–39. [Google Scholar]
- 36.Hokkanen B., Bhatnagar A., Koistinen A., Kangas T., Lassi U., Sillanpää M. Comparison of adsorption equilibrium models and error functions for the study of sulfate removal by calcium hydroxyapatite microfibrillated cellulose composite. Environ. Technol. 2017;39(8):1–63. doi: 10.1080/09593330.2017.1317839. [DOI] [PubMed] [Google Scholar]
- 37.Igwegbe C.A., Onyechi P.C., Onukwuli O.D., Nwokedi I.C. Adsorptive treatment of textile wastewater using activated carbon produced from Mucuna pruriens seed shells. World J. Eng. Technol. 2016;4:21–37. doi: 10.4236/wjet.2016.41003. [DOI] [Google Scholar]
- 38.Chen C. Evaluation of equilibrium sorption isotherm equations. Open Chem. Eng. J. 2013;7:24–44. doi: 10.2174/1874123101307010024. [DOI] [Google Scholar]
- 39.Ayawei N., Ebelegi A.N., Wankasi D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017;2017 doi: 10.1155/2017/3039817. 11. [DOI] [Google Scholar]
- 40.Igwegbe C.A., Al-Rawajfeh A.E., Al-Itawi H.I., Al-Qazaqi S., Hashish E.A., Al-Qatatsheh M., Sharadqah S., Sillanpaa M. Utilization of calcined gypsum in water and wastewater treatment: removal of phenol. J. Ecol. Eng. 2019;20(7):1–10. doi: 10.12911/22998993/108694. [DOI] [Google Scholar]
- 41.Igwegbe C.A., Mohmmadi L., Ahmadi S., Rahdar A., Khadkhodaiy D., Dehghani R., Rahdar S. Modeling of adsorption of Methylene Blue dye on Ho-CaWO4 nanoparticles using Response surface methodology (RSM) and Artificial neural network (ANN) techniques. MethodsX. 2019;6:1779–1797. doi: 10.1016/j.mex.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue F., Wang F., Chen S., Ju S., Xing W. Adsorption equilibrium, kinetics, and thermodynamic studies of cefpirome sulfate by using macroporous resin. Chem. Eng. Data. 2017;62(12):4266–4272. doi: 10.1021/acs.jced.7b00629. [DOI] [Google Scholar]
- 43.Igwegbe C.A., Banach A.M., Ahmadi S. Adsorption of Reactive blue 19 from aqueous environment on magnesium oxide nanoparticles: kinetic, isotherm and thermodynamic studies. Pharm. Chem. J. 2018;5(5):111–121. [Google Scholar]
- 44.Igwegbe C.A., Rahdar S., Rahdar A., Mahvi A.H., Ahmadi S., Banach A.M. Removal of fluoride from aqueous solution by nickel oxide nanoparticles: equilibrium and kinetic studies. Fluoride. 2019;52(4):569–579. [Google Scholar]
- 45.Akbari H., Jorfi S., Mahvi A.H., Yousefi M., Balarak D. Adsorption of fluoride on chitosan in aqueous solutions: determination of adsorption kinetics. Fluoride. 2018;51:319–327. [Google Scholar]
- 46.Abdisa G.J. Preparation and evaluation of adsorption effectiveness of peanut husk for the removal of fluoride ion from aqueous solution. Mod. Chem. Appl. 2018;6:261. doi: 10.4172/2329-6798.1000261. [DOI] [Google Scholar]