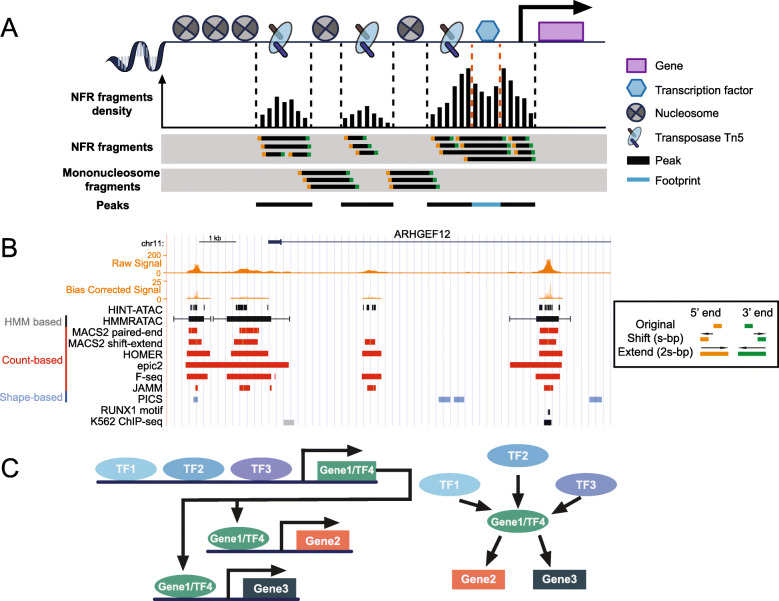
Fig. 3.
Schematic and real ATAC-seq data from core and advanced analyses. a In an ATAC-seq experiment, Tn5 binds and cuts open chromatin and simultaneously ligates adapters. The fragments are sequenced to identify open chromatin regions (black) and footprints (blue). NFR fragments represent the open chromatin, while nucleosome-bound fragments reflect nucleosome positions (gray shaded tracks). b Real ATAC-seq data. Signal tracks are generated from BAM file (Raw) and bias corrected by HINT-ATAC (Bias corrected). Peak sets are generated from three types of peak callers, count-based (red), shape-based (blue), and HMM based (black). For MACS2, two strategies (paired-end and shift-extend) are used. For HMMRATAC, the extended ranges at both sides indicate the nucleosomes. The HINT-ATAC track is footprints detected by HINT-ATAC, while the RUNX1 motif track is the footprints matching RUNX1 motif from JASPAR database. The K562 ChIP-seq track is the RUNX1 ChIP-seq from ENCODE, indicating the footprint detection can recapitulate the real TF binding. The right box illustrates the shift-extend approach. First, it shifts both ends s-bp towards outside, and then extend 2s-bp towards inside. c Illustration of network reconstruction by ATAC-seq data. The presence of TF can be represented by motifs or footprints detected by aforementioned methods. NFR: nucleosome-free region; TF: transcription factor; HMM: hidden Markov model