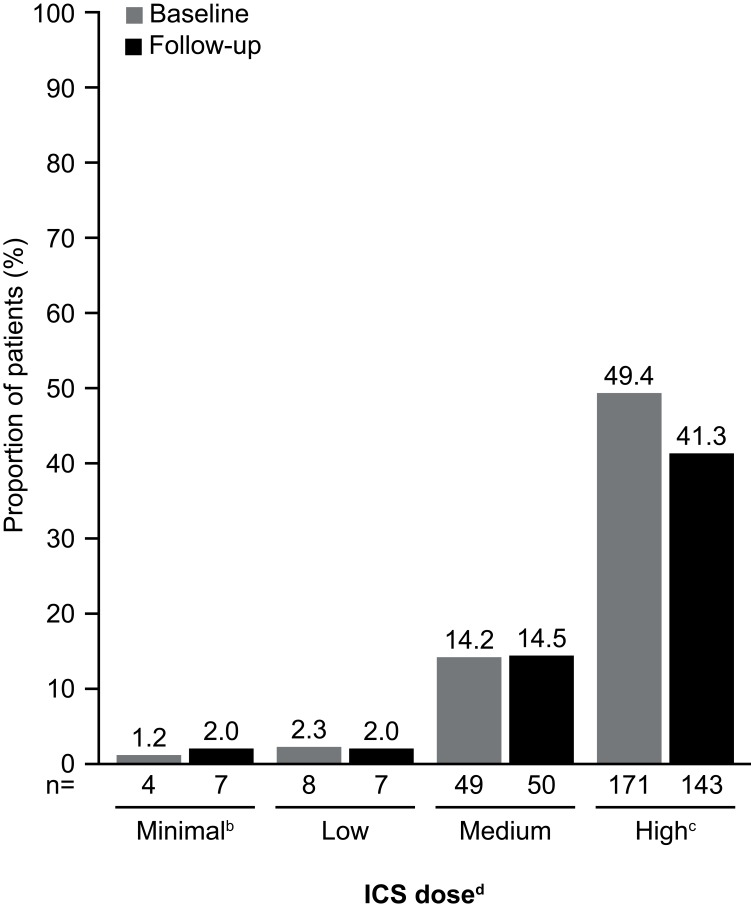
Figure 6.
Use of ICS during the baseline and follow-up periodsa. aBaseline period defined as the 12 months before first mepolizumab treatment; follow-up period defined as the 12 months following first mepolizumab treatment. bPatients on a lower dose than the ‘low” category, or on ICS but missing dosage information on the claims. cP=0.033. dICS dosing categories (low, medium, and high) were determined according to GINA guidelines;1 ICS/LABA combination prescriptions were included. ICS dose was determined based on ICS utilization within the last 90 days before the end of the baseline and follow-up periods. Prescription fills that overlapped into these periods were included in the calculations. N numbers are the numbers of patients with data available. The proportion of patients not on ICS or with data missing was 32.9% (n=114) and 40.2% (n=139) at baseline and follow-up, respectively. Patients were not on ICS in the last 90 days of the baseline or follow-up periods.
Abbreviations: GINA, Global Initiative for Asthma; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist.