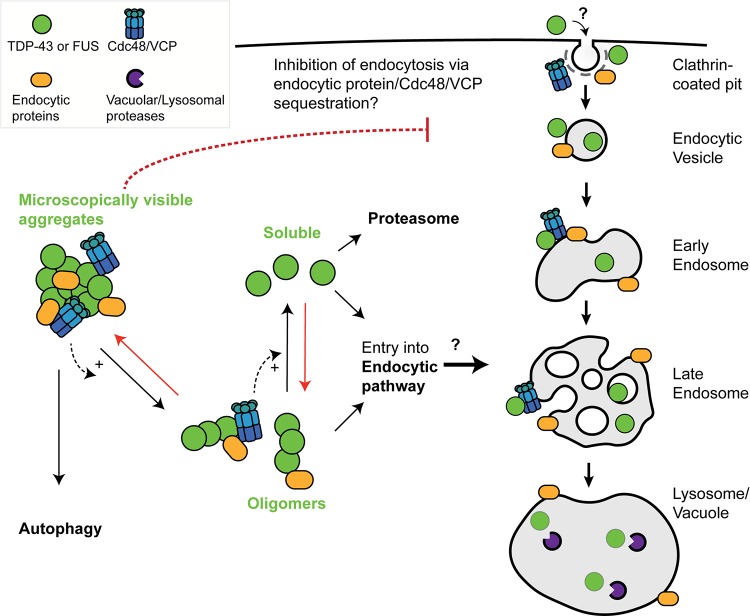
FIG 7.
Speculative model of TDP-43 and FUS engagement with endocytosis and Cdc48/VCP function. In yeast, the toxicity, turnover, and aggregation of both TDP-43 and FUS depend on endocytosis function (26; this study). We suggest that large microscopically visible aggregates of TDP-43 and FUS are most likely cleared from the cytoplasm by autophagy, with oligomeric or soluble forms of TDP-43 and FUS being subject to clearance by endocytic or proteasomal means. If true, given our yeast toxicity data, in which endocytic but not autophagic mutants enhance TDP-43 and FUS toxicity, this would suggest that oligomeric rather than microscopically visible forms of TDP-43 and FUS are the toxic cellular species. Cdc48/VCP, which localizes in microscopically visible TDP-43 aggregates, may facilitate TDP-43 and FUS conversion into oligomeric and soluble forms and/or facilitate entry of TDP-43 and FUS into the endocytic pathway. Red arrows broadly represent antagonistic aggregation-promoting processes, which may include cellular stress, impaired proteostasis, RNA metabolism defects, and perturbation of nuclear-cytoplasmic trafficking, all of which are implicated in ALS pathology. Finally, sequestration of Cdc48/VCP (and various endocytic proteins) within TDP-43 or FUS aggregates may contribute to the observed inhibition of endocytosis rates caused by TDP-43 or FUS expression, owing to various roles described for Cdc48/VCP in the endocytic pathway. Alternatively, TDP-43 and FUS may directly impair endocytic trafficking by affecting endocytic vesicle invagination, fusion, or other trafficking processes. How TDP-43 and FUS inhibit endocytosis and the means by which TDP-43 and FUS enter the endocytic pathway remain mechanistically undefined.