Figure 8.
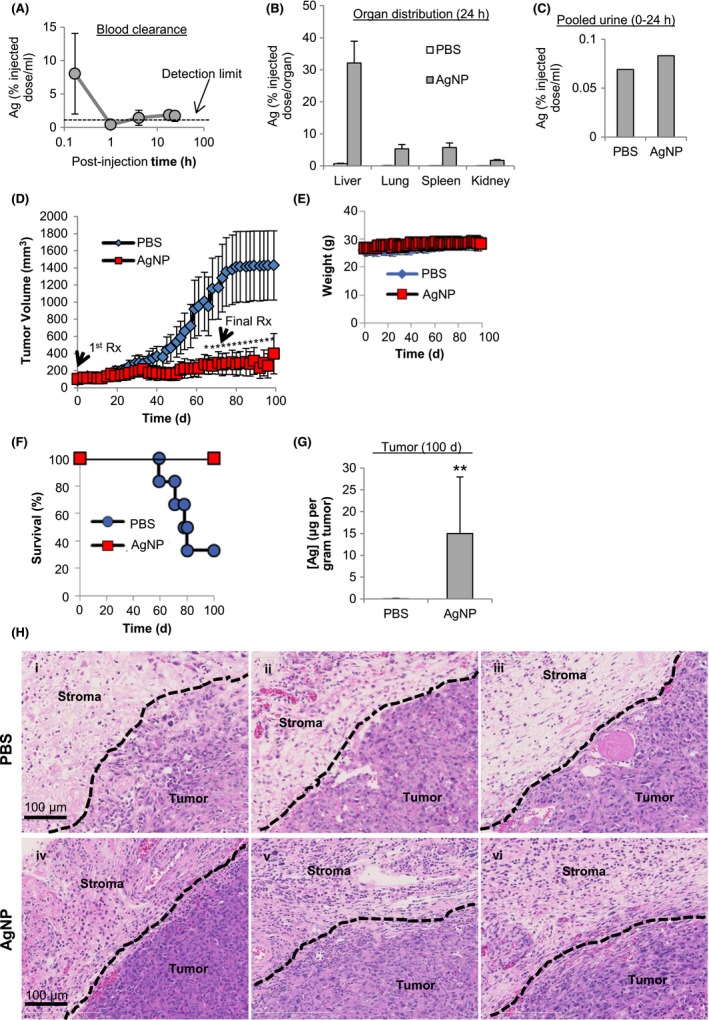
Safety and efficacy of intravenous delivery of AgNPs for treatment of triple‐negative breast cancer tumors in vivo. (A) Blood clearance, (B) biodistribution, and (C) urine content of AgNPs (6 mg/kg; single dose; IV) were quantified in nude mice by ICP‐MS after 24 h for 20 µL of blood, the entire organ, or entire volume of collected urine. (D) MDA‐MB‐231 tumor bearing nude mice were intravenously injected with PBS (6 mice) or AgNPs (7 mice; 6 mg/kg; 3× per wk; 10 wks) and tumor growth was quantified over time by calipers. (E) Mice were monitored for weight change. Statistical analysis was performed using two‐way ANOVA and post‐hoc Tukey test. Significant differences in tumor size are indicated (*P < .05). (F) Survival of treated mice is plotted by Kaplan‐Meyer analysis. Due to tumor growth in excess of the severity limit of the protocol (>1000 mm3) 2/3 of PBS treated mice were euthanized prior to the end of the study at 100 d. No tumors in AgNP treated mice reached the tumor size limit and all mice survived until the completion of the study at 100 d. (G) Silver content in residual tumors of AgNP treated mice and PBS treated mice was quantified by ICP‐MS. Statistical analysis was performed by student t‐test. A significant difference in silver content is indicated (**P < .01). (H) Hematoxylin and eosin stained sections of tumor and adjacent stroma from individual PBS (panels i‐iii) and AgNP (panels iv‐vi) treated mice are shown
