Figure 7.
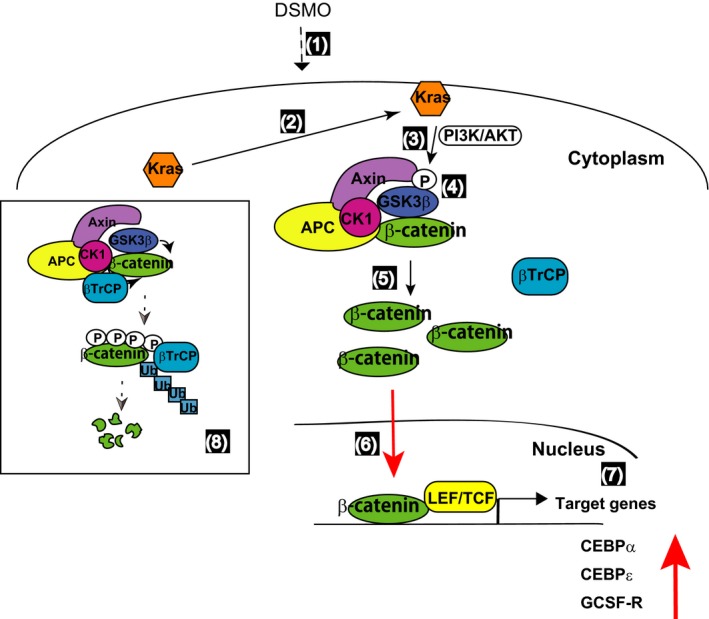
Novel Kras‐Wnt/β‐catenin signaling networks in the DMSO‐induced differentiation of HL‐60 cells. DMSO triggers Kras activation through the recruitment of Kras to the plasma membrane (1, 2). AKT phosphorylates GSK3β and inactivates GSK3β (3, 4). Accumulated unphosphorylated β‐catenin in the cytoplasm translocates to the nucleus (5, 6), where it binds to Lef/Tcf and stimulates the transcription of target genes such as C/EBPα, C/EBPɛ, and G‐CSF receptor (7). In the absence of DMSO, β‐catenin is phosphorylated by a destruction complex composed of the core proteins, including Axin, APC, CK1, β‐catenin, GSK3β, and E3‐ubiquitin ligase β‐TrCP. Degradation of phosphorylated β‐catenin follows its ubiquitination by proteasomes (8)
