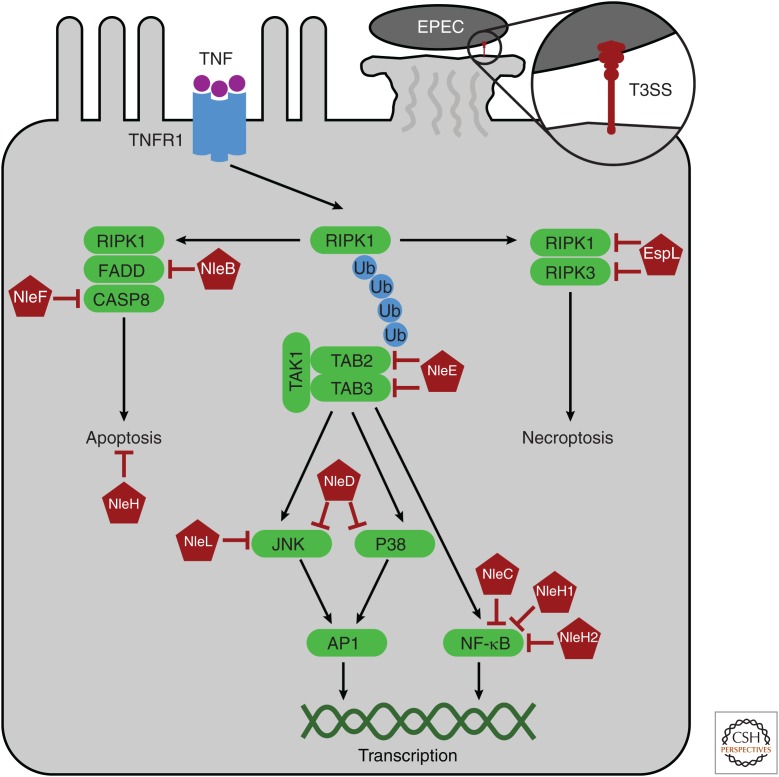
Figure 4.
Enteropathogenic Escherichia coli (EPEC) virulence factors target pathways for transcription, apoptosis, and necroptosis. Tumor necrosis factor (TNF) receptor signaling can trigger one of three responses. The cell will first try to initiate transcriptional signaling pathways. RIPK1 acts as a scaffold for polyubiquitin chains, on which assemble TAB2 and TAB3 in a complex with TAK1. Downstream from TAK1, JNK and p38 signal to the transcription factor AP1, and other complexes signal to nuclear factor (NF)-κB. Transcription can be blocked by EPEC effectors: NleE inhibits TAB2 and TAB3, NleD obstructs JNK and p38, and NleC and NleH1/2 block NF-κB. When transcription pathways are hindered, guards of RIPK1 initiate apoptosis. RIPK1 death domain (DD) becomes exposed allowing homotypic interactions with the DD of the adaptor FADD, which in turn recruits caspase-8 to initiate apoptosis. EPEC has additional virulence strategies to inhibit the apoptotic guard pathway: NleB and NleF attack FADD and caspase-8, respectively. Finally, the host cell has a third guard pathway in which RIPK1 uses its integrated guard RHIM and kinase domains to activate the guard, RIPK3. RIPK3 signaling then induces necroptosis of the host cell. Similar to transcription and apoptosis, EPEC has a virulence factor, EspL, to inhibit signaling to necroptosis.