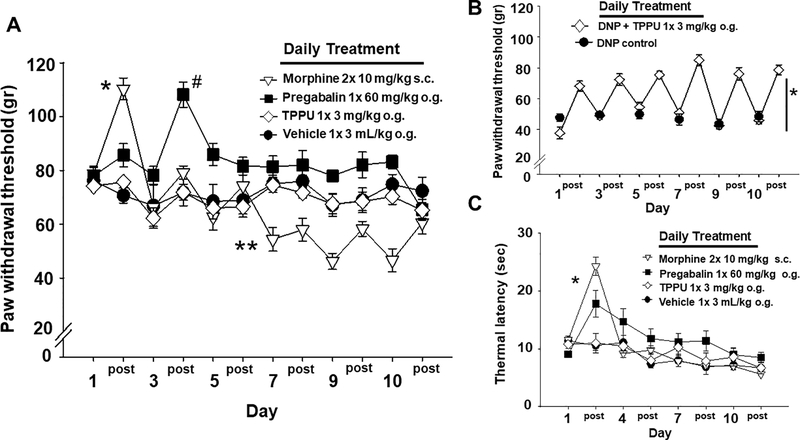
Figure 1.
TPPU mediates effective analgesia without tolerance. A) Tolerance develops with opioids and gabapentinoids. Initial doses of morphine dramatically raised mechanical withdrawal thresholds (MWTs) in naïve rats. The pre- and 60 min post-treatment measures per day depicted in the graph show the diminishing response to treatment over 10 days with morphine indicating tolerance with significant increases on Day 1 (* p ≤ 0.001) and decreases on Day7 (** p=0.005) suggesting the development of opioid induced hyperalgesia. Pregabalin also increased MWT but later (Day 3, # p ≤ 0.001), after which subsequent dosing had no effect but did not lower MWT below baseline. TPPU was indistinguishable from vehicle in this assay in naïve animals. TPPU did not alter MWT early in the time course and it also did not induce any apparent hyperalgesia with repeated dosing. B) In rats with diabetic neuropathic pain (DNP), TPPU significantly increased MWTs over the 10 days of treatment compared to controls (* p ≤ 0.001). The analgesic response from pre-treatment (numbered day) to measures assessed 60 min post treatment (‘post’ per numbered day) did not diminish with repeated administration of the sEHI indicating that tolerance did not develop. C) TPPU dosed to healthy rats does not alter thermal sensitivity. In contrast, both morphine and pregabalin significantly (* p ≤ 0.001) increased the hotplate (52.5°C) latency early in the time course (Day 1) compared to the vehicle indicating an initial response which diminished with repeated dosing (Days 4, 7, 10). The sEHI TPPU showed no change compared to vehicle over the entire time course. DNP rats loose thermal sensitivity altering responses and therefore were not assessed with the thermal assay.