Figure EV4. Non‐phosphorylatable substitutions of T142 in ASY1 reduce its interaction strength with ASY3.
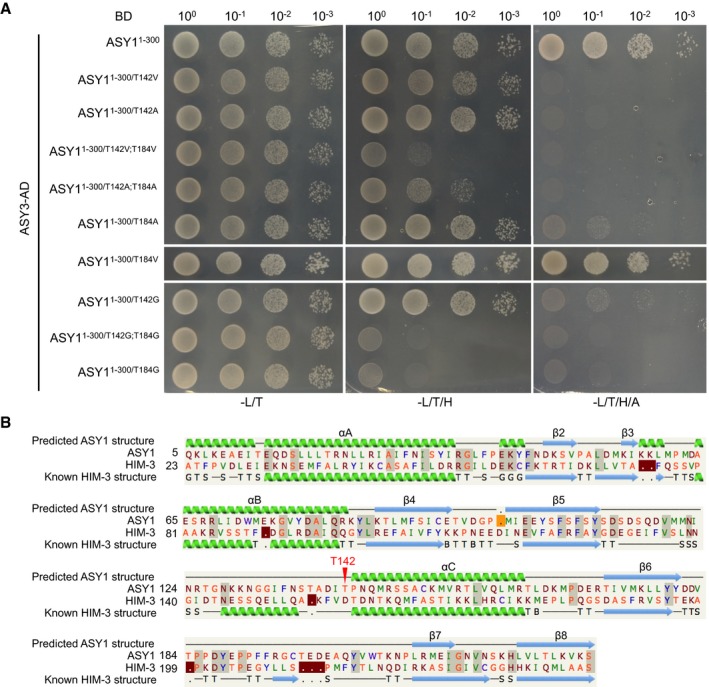
- Yeast two‐hybrid interaction assays of ASY3 with different ASY1 variants. Yeast cells harboring both the AD and BD plasmids were grown on synthetic medium supplied with glucose in the absence of Leu and Trp (‐L/T, left panel), on SD medium in the absence of Leu, Trp, and His (‐L/T/H, middle panel), and on SD medium in the absence of Leu, Trp, His, and Ade (‐L/T/H/A, right panel). Yeast cells were incubated until OD600 = 1 and then diluted 10‐, 100‐, and 1,000‐fold for the assays.
- The predicted structure of ASY1 HORMA domain based on the known structure of C. elegans HIM‐3 (c4trkA) using Phyre2 protein structure prediction. Red arrowhead indicates the T142 site of ASY1.
