Abstract
Background
Cancer is the second leading cause of death globally, causing an estimated 9.6 million deaths in 2018. Low cancer symptom awareness has been associated with poor cancer survival for all cancers combined. The Cancer Awareness Measure (CAM) is a validated, face-to-face survey used since 2008 to measure the UK public’s awareness of the symptoms and risk factors of cancer as well as the barriers to seeking help.
Objective
The aim of this study is to explore whether online data collection can produce a representative sample of the UK population, compare awareness of cancer signs and risk factors and the barriers to seeking help between data collected online and face-to-face, and examine the relationships between awareness and demographic variables.
Methods
Differences in awareness of cancer signs, symptoms, and risk factors among samples were explored while adjusting for demographic differences (age, gender, ethnicity, educational level, marital status, and country of residence) to distinguish the effect of data collection method. Multivariate logistic regression models were used to calculate adjusted odds ratios for recall and recognition of signs and symptoms, risk factors, and barriers to seeking help.
Results
A total of 4075 participants completed the CAM, 20% (n=819) via face-to-face interviews and 80% online (n=3256; agency A: n=1190; agency B: n=2066). Comparisons of data collected using face-to-face interviews and online surveys revealed minor differences between samples. Both methods provided representative samples of the UK population with slight differences in awareness of signs, symptoms, and risk factors and frequency of help-seeking barriers reported.
Conclusions
These findings support a move to online data collection for the CAM. The flexibility afforded will enable the CAM to explore a wider range of issues related to the prevention, early diagnosis, and treatment of cancer.
Keywords: neoplasms, surveys and questionnaires, cross-sectional study, awareness, help-seeking behavior, cancer
Introduction
Background
Cancer is the second leading cause of death globally, causing an estimated 9.6 million deaths in 2018 [1]. Half of the people diagnosed with cancer in England and Wales survive for 10 years or more, but approximately 4 in 10 cases of cancer in the UK could be prevented [2]. Cancer survival has consistently been reported to be lower in the UK than similar European countries [3,4].
Late-stage diagnosis contributes to excess deaths for bowel [5], breast [6], and lung cancer [7] in the UK. Late diagnosis could be related to low awareness of symptoms, leading to delays in seeking medical help. Low cancer symptom awareness has been associated with delays in seeking medical help and poor cancer survival for all cancers combined [8].
Earlier detection can improve patient experience [9], costs to the National Health Service (NHS) [10], and cancer survival, but it relies partly on prompt presentation [11]. Understanding and potentially improving awareness of cancer signs is an important step in reducing the incidence of late-stage cancer and reducing cancer deaths in the UK.
In 2008, Cancer Research UK, in partnership with University College London, King’s College London, and University of Oxford, developed the Cancer Awareness Measure (CAM) [12]. The CAM is a validated survey designed to measure awareness of signs, symptoms, and risk factors for cancer and potential barriers to seeing a doctor.
Cancer Research UK has used the CAM to collect data biannually from 2008 to 2014 from a representative sample of the UK population via the Office for National Statistics (ONS) Opinions and Lifestyle Survey. Questions in the survey are a combination of recall and recognition questions, designed to assess public awareness. Recall questions are open-ended questions, asking participants to list as many cancer warning signs and risk factors that they can think of. These are followed by recognition questions, where participants are given a list of warning signs and risk factors and asked yes/no do they think these are risk factors or warning signs of cancer.
Data from the CAM indicate that the average number of cancer warning signs recognized by representative samples of the UK population has increased from 6.4 (SD 1.9) in 2008 to 6.8 (SD 1.5) in 2014 out of a possible nine warning signs posed in the survey [13]. Recall of risk factors appears to have followed the opposite pattern, with recall decreasing from a mean 2.2 in 2008 to 2.0 in 2014 [13]. Awareness of cancer signs and risk factors has consistently been found to be lower among men [14,15], younger adults [14], and those from lower socioeconomic groups [14,16,17] or ethnic minorities [15,18].
Although CAM data have traditionally been collected via face-to-face interviews conducted by the ONS, the response rates have declined over the years (from 61% in 2008 to 47% in 2017). This study explores the viability of moving data collection online, a move seen in many large market research organizations. In Great Britain, 90% of households have access to the internet, and 73% of people have accessed the internet with a mobile phone [19]. The benefits of online data collection include lower costs [17], higher data quality [20], and a faster rate of return and lower data entry times [21]. Conversely, the limitations may include sampling issues [21] and differences in sampling methodologies [22].
Although the relationships among questionnaire modality, response rates, and accuracy have been described as complex [23], previous research exploring the impact of data collection method is encouraging. Socially desirable behaviors have been reported to be less likely to be disclosed in interviews than online questionnaires [24], and disease prevalence rates are much closer to known rates when using internet studies compared with data collected over the telephone or face-to-face [25].
Research Objectives
The primary aim of this study is to identify the extent to which public awareness of cancer and attitudes toward seeking help vary by data collection method (face-to-face vs online data) in adults (aged ≥18 years) in Great Britain. The research objectives are to (1) explore whether online data collection can produce a representative sample of the UK population (differences between samples); (2) compare the awareness of signs, symptoms, and risk factors for cancer, as well as the barriers to seeking help between data collected online and face-to-face (differences in levels of awareness); and (3) explore whether any relationships observed between awareness and demographic variables are consistent across samples (interactions between survey provider and demographic variables).
Methods
Participants and Recruitment
Face-to-Face Sample
Between January and March 2017, face-to-face data were collected by the ONS via the Opinions and Lifestyle survey. The ONS use stratified probability sampling to select sampling points from a database of 27 million private households in the UK. A random sample of addresses from each sampling point were selected, and interviewers invited one adult respondent from each household to complete the CAM using a face-to-face, computer-assisted interview.
Online Samples
Online samples were recruited by two market research agencies. Agency A recruited participants to their online panel via a face-to-face survey. Agency A used a probability-based approach for recruitment, which avoids in-built bias commonly found in online panel sampling methods. Agency B used “active sampling,” in which a subsample of participants were selected from their more than 800,000-member panel based on their age, gender, social class, and education. Agency B panel members are recruited from standard advertising and strategic partnerships with a range of websites.
Great Britain Population Data
The Great Britain population statistics were taken from the ONS (midyear population estimates, Households and Individuals Internet Access survey), census data, and NHS Digital (Health Survey for England).
Outcome Measures
Variables collected in the CAM are outlined in Textbox 1. Details of the development and content of the CAM can be found elsewhere [12].
Outcome measures.
Sociodemographic characteristics
We amended the standard ONS demographic questions and adapted these for online samples where necessary: age, gender, educational attainment, ethnicity, country of residence marital status, internet use, and self-reported health status.
Awareness of signs and symptoms of cancer (recall and recognition)
Recall: “There are many warning signs and symptoms of cancer, please name as many as you can think of.”
Recognition: “Could any of the following be signs of cancer?”: lump or swelling, persistent unexplained pain, unexplained bleeding, persistent cough or hoarseness, persistent change in bowel or bladder habits, difficulty swallowing, change in the appearance of a mole, a sore that does not heal, and unexplained weight loss.
Awareness of cancer risk factors (recall and recognition)
Recall: “What things do you think affect a person’s chance of developing cancer?”
Recognition: “Could any of the following increase a person’s chance of developing cancer?”: smoking, getting sunburned, exposure to another person’s smoking, drinking alcohol, having a close relative with cancer, being overweight, being older, not eating many fruits and vegetables, not eating enough fiber, eating too much red or processed meat, not doing much physical activity, and infection with HPV (human papillomavirus).
Barriers to seeing a general practitioner
“Which of the following might put you off going to the doctor?”
Participants were asked to indicate whether any of a range of barriers might put them off seeing a doctor on a 5-point agreement scale from strongly agree to strongly disagree.
To reduce bias, open-ended questions about signs, symptoms, and risk factors were asked before closed questions. The number of warning signs endorsed or risk factors recognized were summed to produce total scores. Coding manuals were provided to all market research agencies regarding how to code recalled items to ensure consistency.
Statistical Analysis
Weighting and Sample Differences
Each market research agency provided their own weighting variable to ensure the sample was representative of the Great Britain population and to adjust for nonresponse where possible. Our analyses were carried out using the weighted variable provided by each agency. We did not create a bespoke weighting variable because of the lack of nonresponse data available. See Multimedia Appendix 1 for how each survey provider weighted their data.
Weighted sample demographics were compared between the surveys to explore any differences between the collected samples. Differences between survey responses and Great Britain population statistics were not tested for significance because confidence intervals for Great Britain data were not available.
Differences in Levels of Awareness
Differences in awareness of cancer signs and symptoms and risk factors between samples were explored while adjusting for demographic differences (age, gender, ethnicity, educational level, marital status, and country of residence) with the aim of determining the effect of data collection method.
Multivariate logistic regression models were used to calculate adjusted odds ratios for recall and recognition of signs and symptoms, risk factors, barriers to seeking help, and awareness of bowel screening. The outcome variable was binary to show if the responder did or did not recall or recognize signs and symptoms, risk factors, barriers to seeking help, and awareness of bowel screening. Only statistically significant variables were included in the final logistic regression models.
Interactions Between Outcomes and Demographic Variables
Interaction terms between survey provider and key demographics (gender, age, education level, marital status, ethnicity, country, long-term health, and internet usage) were added to the awareness models. Whether data collected by different methods varied by demographic variables, while controlling for any differences in sample characteristics between the surveys, was explored.
Results
Participants
In total, 4075 participants completed the CAM. Online participants made up 80% (n=3256) of the sample (agency A: n=1190; agency B: n=2066). The remaining 20% (n=819) of participants completed face-to-face interviews.
Differences Between Samples
The three weighted samples were generally representative of the Great Britain population (Table 1).
Table 1.
Demographic characteristics by survey provider and compared with Great British population statistics (N=4075).
| Demographic | Face-to-face, % | Online, %a | Great Britain population, % | ||
|
|
Office for National Statistics (n=819) | Agency A (n=1190) | Agency B (n=2066) |
|
|
| Age groups |
|
|
|
|
|
|
|
18-24 | 10.2 | 8.4 | 12.0 | 15.1b |
|
|
25-44 | 33.8 | 33.4 | 32.1 | 32.1 |
|
|
45-54 | 18.0 | 17.9 | 20.9 | 17.2 |
|
|
55-64 | 15.1 | 16.9 | 16.9 | 13.9 |
|
|
≥65 | 22.8 | 23.1 | 18.1 | 21.7 |
|
|
Missing | — | 0.2 | — | — |
| Gender |
|
|
|
|
|
|
|
Male | 49.1 | 49.9 | 48.0 | 49.3 |
|
|
Female | 50.9 | 50.1 | 52.0 | 50.7 |
| Ethnicity |
|
|
|
|
|
|
|
White | 87.9 | 87.5 | 92.7 | 86.0 |
|
|
Nonwhite | 12.1 | 12.5 | 7.3 | 14.0 |
| Country of residence |
|
|
|
|
|
|
|
England | 86.6 | 84.7 | 86.3 | 86.5 |
|
|
Scotland | 8.3 | 10.2 | 8.7 | 8.6 |
|
|
Wales | 5.1 | 5.1 | 5.0 | 4.9 |
| Higher education qualification |
|
|
|
||
|
|
Degree | 30.5 | 26.4 | 32.2 | 27.1 |
|
|
Below degree | 42.7 | 55.7 | 54.1 | 44.7 |
|
|
No qualifications | 12.7 | 15.5 | 6.6 | 23.0 |
|
|
Other | 14.1 | 2.3 | 5.5 | 5.2 |
|
|
Don’t know | — | — | 1.6 | — |
| Marital status |
|
|
|
|
|
|
|
Partner | 50.5 | 62.6 | 61.7 | 50.9 |
|
|
No partner | 49.5 | 37.4 | 38.3 | 49.1 |
| Long-term illness |
|
|
|
|
|
|
|
Very good | 37.0 | 20.1 | 15.6 | Very good/good: 76 |
|
|
Good | 42.0 | 48.5 | 47.4 | — |
|
|
Fair | 15.9 | 23.7 | 28.3 | — |
|
|
Bad | 3.6 | 6.4 | 7.1 | Very bad/bad: 7 |
|
|
Very bad | 1.3 | 1.3 | 1.6 | — |
|
|
Refused | 0.3 | 0.1 | — | — |
| Internet usage |
|
|
|
|
|
|
|
Several times a day | 64.2 | 65.6 | 79.9 | At least once a day: 80 |
|
|
Once a day | 14.3 | 13.2 | 13.7 | — |
|
|
4-6 days a week | 3.1 | 2.9 | 3.0 | — |
|
|
2-3 days a week | 3.7 | 4.2 | 1.6 | — |
|
|
Once a week | 2.1 | 2.4 | 0.6 | At least weekly: 8 |
|
|
Less than once a week | 1.3 | 2.2 | 0.4 | Less than weekly: 2 |
|
|
Never | 9.2 | 9.5 | 0.8 | Did not use in the last 3 months: 10 |
|
|
Don’t know | 0.9 | — | — | — |
|
|
Refused | 1.3 | — | — | — |
aPercentages are weighted using the weighting variable provided by each survey agency; see Multimedia Appendix 1 for more information.
bAges 15-24 years.
The gender split of all three samples largely matched the Great Britain population; however, both online samples were older than the ONS sample and the Great Britain population. Scottish participants were slightly overrepresented by agency A (10.2% vs 8.6% of Great Britain population).
All samples included a higher proportion of white participants than the Great Britain population (Great Britain population: 86%; agency A: 87.5%; agency B, 93%) and reported higher educational attainment. Both online samples had a larger proportion of participants with a partner (agency A: 63%; agency B: 62%) compared with the Great Britain population (50.9%) and were more likely to report being in good health (agency A: 48.5%; agency B: 47.4%; ONS: 42%). Face-to-face participants were less likely to report their health as bad (3.6%; agency A: 6.4%; agency B: 7.1%; Great Britain population: 7%). More than 90% of agency B participants reported using the internet more than once a day compared with 78.5% of face-to-face and 78.8% of agency A participants.
Differences in Levels of Awareness (Outcomes)
The number of cancer warning signs and risk factors recognized and recalled within each sample are included in Multimedia Appendix 2.
Cancer Warning Signs
Recall of Warning Signs
Agency A participants recalled significantly more signs of cancer than other participants, with a mean recall of five signs of cancer compared with three for both face-to-face and agency B participants. Figure 1 shows the percentage of participants recalling cancer warning signs.
Figure 1.
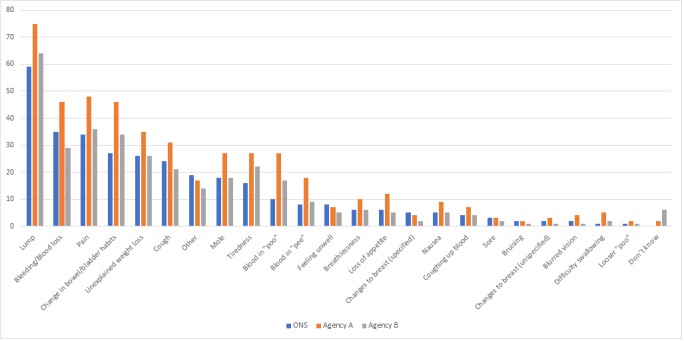
Percentage of participants recalling cancer warning signs.
A lump was the most frequently recalled sign in all three samples (agency A: 75.1%, agency B: 64.2%, face-to-face: 58.6%; Figure 1). Compared with face-to-face participants, agency B participants were less likely to recall bleeding or blood loss (29% vs 35%, P<.001, OR 0.7, 95% CI 0.6-0.8) and sores (1.5% vs 2.7%, P=.003, OR 0.4, 95% CI 0.2-0.8). Agency A participants were more likely than face-to-face participants to recall a lump (75% vs 59%, P<.001, OR 2.3, 95% CI 1.8-2.8), pain (48% vs 34%, P<.001, OR 1.9, 95% CI 1.6-2.3), bleeding or blood loss (46% vs 35%, P<.001, OR 1.5, 95% CI 1.3-1.8), and blood in urine (18% vs 8%, P<.001, OR 2.5, 95% CI 1.9-3.3). Participants from both online samples were more likely than face-to-face participants to recall change in bowel or bladder habits (agency A: 46%, P<.001, OR 2.9, 95% CI 2.4-3.5; agency B: 34%, P<.001, OR 1.5, 95% CI 1.2-1.8 vs face-to-face: 27%), blood in feces (agency A: 26%, P<.001, OR 4.2, 95% CI 3.3-5.6; agency B: 17%, P<.001, OR 2.1, 95% CI 1.6-2.7 vs face-to-face: 9.6%) and tiredness (agency A: 28%, P<.001, OR 2.1, 95% CI 1.7-2.7; agency B: 22%, P=.04, OR 1.3, 95% CI 1.0-1.6 vs face-to-face: 16%). Online samples were more likely to answer “don’t know” when asked to recall warning signs for cancer than face-to-face responders (agency A: 1.8%, P=.02, OR 4.4, 95% CI 1.5-19.3; agency B: 6.1%, P<.001, OR 20.4, 95% CI 7.5-38.5; face-to-face: 0.2%).
Recognition of Cancer Signs
Agency A participants demonstrated greater recognition of signs and symptoms, recognizing a mean of eight of nine presented signs and symptoms of cancer, compared with ONS and agency B participants who recognized a mean of seven.
An unexplained lump or swelling was the most commonly recognized sign in all samples (face-to-face: 94.7%; agency A: 98.4%; agency B: 94.7%; Table 2). Agency A participants were more likely than face-to-face participants to recognize a lump (98% vs 95%, P=.009, OR 2.4, 95% CI 1.3-4.6) and unexplained weight loss (96% vs 89%, P<.001, OR 3.5, 95% CI 2.3-5.5). For other signs, there were no significant differences between agency A and face-to-face responses.
Table 2.
Percentage of participants from each sample who answered “yes” to the question “Do you think that the following could be a warning sign for cancer?” (N=4075).
| Yes, it could | Face-to-face, % | Online | |||
|
|
Office for National Statistics (n=819) | Agency A (n=1190) | Agency B (n=2066) | ||
|
|
|
Participants, % | P value | Participants, % | P value |
| Unexplained lump or swelling | 94.7 | 98.4 | .009 | 94.7 | .002 |
| Change in appearance of a mole | 92.9 | 95.9 | .09 | 93.9 | .006 |
| Persistent change in bowel or bladder habits | 89.8 | 91.4 | .58 | 88.2 | .001 |
| Unexplained weight loss | 89.1 | 96.4 | <.001 | 86.5 | .03 |
| Unexplained bleeding | 88.0 | 89.1 | .93 | 86.3 | .005 |
| Persistent cough or hoarseness | 83.7 | 86.7 | .10 | 82.8 | .01 |
| Persistent unexplained pain | 79.0 | 82.0 | .05 | 83.8 | .47 |
| Persistent difficulty swallowing | 78.3 | 76.3 | .15 | 76.2 | .004 |
| Sore that does not heal | 63.0 | 66.6 | .18 | 70.0 | .01 |
Agency B participants were less likely than face-to-face participants to recognize a lump (94% vs 95%, P=.002, OR 0.4, 95% CI 0.3-0.7), changes in bowel habits (88% vs 90%, P<.001, OR 0.5, 95% CI 0.4-0.7), persistent cough (83% vs 84%, P=.01, OR 0.7, 95% CI 0.6-0.9), unexplained weight loss (87% vs 89%, P=.03, OR 0.7, 95% CI 0.5-0.9), persistent difficulty swallowing (76% vs 78%, P=.004, OR 0.7, 95% CI 0.6-0.9), and unexplained bleeding (86% vs 88%, P=.005, OR 0.7, 95% CI 0.4-0.9). Agency B participants were more likely to recognize a sore that does not heal as a sign or symptom of cancer (70% vs 63%, P=.01, OR 1.3, 95% CI 1.1-1.5).
Awareness of Risk Factors for Cancer
Recall of Cancer Risk Factors
Agency A participants recalled a mean of five risk factors compared with both face-to-face and agency B participants who recalled a mean of three. Fewer agency A participants recalled zero risk factors (3.2%) than face-to-face (8.2%) or agency B (11.6%) participants (Multimedia Appendix 1).
The most frequently recalled risk factor within all samples was smoking, but recall was significantly lower in the agency B sample (P<.001, OR 0.4, 95% CI 0.3-0.5; Table 3). The same pattern was seen for alcohol (agency A: 55%, P=.07, OR 1.2, 95% CI 1.0-1.4; face-to-face: 54%; agency B: 43%, P<.001, OR 0.7, 95% CI 0.6-0.8). A higher proportion of agency B participants answered “don’t know” to this recall question (5.4%, P<.001; face-to-face: 0.1%; agency A: 0.9%).
Table 3.
Recall of risk factors for cancer from the three samples (N=4075).
| Risk factor | Face-to-face, % | Online | |||
|
|
Office for National Statistics (n=819) | Agency A (n=1190) | Agency B (n=2066) | ||
|
|
|
Participants, % | P value | Participants, % | P value |
| Smoking | 81.9 | 81.5 | .96 | 68.6 | .001 |
| Alcohol | 53.5 | 55.1 | .07 | 43.3 | .001 |
| Diet (unspecified) | 36.2 | 50.3 | .001 | 40.0 | .02 |
| Sunburn | 25.0 | 30.1 | .001 | 21.1 | .06 |
| Being overweight | 14.9 | 20.0 | .04 | 25.6 | .001 |
| Exercise | 13.8 | 24.1 | .001 | 16.4 | .06 |
| Occupational exposure | 13.7 | 12.2 | .88 | 8.5 | .001 |
| Genes | 11.5 | 23.8 | .001 | 19.8 | .001 |
| Pollution | 10.4 | 13.0 | .002 | 7.8 | .02 |
| Family history | 10.0 | 22.6 | .001 | 15.2 | .003 |
| Lifestyle | 9.6 | 18.1 | .001 | 14.9 | .02 |
| Stress | 8.4 | 11.7 | .001 | 5.6 | .02 |
| Radiation | 5.9 | 6.3 | .19 | 4.7 | .06 |
| High-fat diet | 4.6 | 2.1 | .001 | 1.0 | .001 |
| Red meat | 3.7 | 3.9 | .51 | 3.3 | .80 |
| Sun beds | 3.7 | 5.3 | .09 | 2.0 | .01 |
| Passive smoking | 2.7 | 3.1 | .07 | 1.5 | .001 |
| Older age | 2.3 | 6.1 | .001 | 4.8 | .009 |
| Mobile phones | 1.1 | 0.3 | .60 | 0.2 | .06 |
| Many sexual partners | 1.0 | 0.6 | .98 | 1.5 | .10 |
| Other | 12.9 | 24.5 | .001 | 12.9 | .61 |
| Nothing | 2.8 | 0.0 | — | 0.4 | — |
| Refused | 4.4 | 0.0 | — | 0.0 | — |
| Don’t know | 0.1 | 0.9 | .05 | 5.4 | .001 |
Recall of sunburn (30%, P<.001, OR 1.7, 95% CI 1.3-2.0), genes (24%, P<.001, OR 2.4, 95% CI 1.8-3.0), and lack of exercise (24%, P<.001, OR 2.1, 95% CI 1.6-2.7) as risk factors was significantly higher in the agency A survey compared with the face-to-face survey (sunburn: 25%; genes: 12%; lack of exercise: 14%). Agency B participants were less likely than face-to-face participants to recall occupational exposure (9% vs 14%, P<.001, OR 0.6, 95% CI 0.5-0.8), stress (6% vs 8%, P=.02, OR 0.6, 95% CI 0.5-0.9), and high-fat diet (1% vs 5%, P<.001, OR 0.2, 95% CI 0.1-0.3). Participants from both online surveys were more likely than face-to-face participants to recall being overweight (agency A: 20%, P=.04, OR 1.4, 95% CI 1.1-1.8; agency B: 25%, P<.001, OR 1.8, 95% CI 1.4-2.2; face-to-face: 15%), family history (agency A: 23%, P<.001, OR 2.8, 95% CI 2.1-3.6; agency B: 15%, P=.003, OR 1.5, 95% CI 1.1-2.0; face-to-face: 10%), lifestyle (agency A: 18%, P<.001, OR 1.8, 95% CI 1.4-2.4; agency B: 15%, P=.02, OR 1.4, 95% CI 1.1-1.8; face-to-face: 10%), diet (agency A: 50%, P<.001 OR 2.2, 95% CI 1.8-2.7; agency B: 40%, P=.02, OR 1.2, 95% CI 1.0-1.4; face-to-face: 36%), and older age (agency A: 6%, P<.001, OR 3.4, 95% CI 2.1-5.9; agency B: 5%, P=.009, OR 2.0, 95% CI 1.2-3.5; face-to-face: 2%) as risk factors for cancer. The only risk factor that face-to-face participants were more likely to recall was having a high-fat diet (face-to-face: 5%; agency A: 2%, P=.001, OR 0.4, 95% CI 0.3-0.7; agency B: 1%, P<.001, OR 0.2, 95% CI 0.1-0.3).
Recognition of Cancer Risk Factors
Online participants recognized more risk factors, a mean of 9 of 12 listed compared with 8 for face-to-face participants.
Participants recruited by online agencies were more likely than face-to-face participants to recognize being overweight (agency B: 74%, P<.001, OR 1.4, 95% CI 1.1-1.7; agency A: 73%, P=.004, OR 1.3, 95% CI 1.1-1.7; face-to-face: 67%), having a family history of cancer (agency B: 77%, P=.02, OR 1.3, 95% CI 1.0-1.5; agency A: 77%, P<.001, OR 1.5, 95% CI 1.2-1.9; face-to-face: 69%), eating too much red or processed meat (agency B: 61%, P<.001, OR 1.5, 95% CI 1.3-1.8; agency A: 58%, P<.001, OR 1.5, 95% CI 1.3-1.8; face-to-face: 52%), and infection with human papillomavirus (HPV) as risk factors of cancer (agency A: 41%, P<.001, OR 1.8, 95% CI 1.5-2.2 agency B: 49%, P<.001, OR 2.4, 95% CI 2.0-2.9; face-to-face: 29%). Agency B participants were more likely than face-to-face participants to recognize older age (68% vs 60%, P<.001, OR 1.4, 95% CI 1.2-1.7) but less likely to recognize smoking (95% vs 96%, P=.001, OR 0.4, 95% CI 0.2-0.7) as risk factors of cancer (Table 4).
Table 4.
Percentage of participants from each sample that recognized each risk factor for cancer (N=4075).
| Risk factor | Face-to-face, % | Online | |||
|
|
ONS (n=819) | Agency A (n=1190) | Agency B (n=2066) | ||
|
|
|
Participants, % | P value | Participants, % | P value |
| Smoking | 96.3 | 98.6 | .24 | 95.4 | .001 |
| Getting sunburned | 94.0 | 94.6 | .40 | 93.7 | .42 |
| Exposure to another person’s smoking | 88.6 | 88.2 | .77 | 86.1 | .002 |
| Drinking alcohol | 78.9 | 78.6 | .43 | 78.6 | .93 |
| Having a close relative with cancer | 68.5 | 76.6 | .001 | 76.6 | .02 |
| Being overweight | 66.6 | 72.8 | .004 | 74.1 | .001 |
| Being older | 60.1 | 57.1 | .16 | 67.8 | .001 |
| Not eating many fruits and vegetables | 52.8 | 53.3 | .95 | 53.6 | .13 |
| Not eating enough fiber | 52.6 | 46.4 | .10 | 49.1 | .37 |
| Eating too much red or processed meat | 51.5 | 57.9 | .001 | 61.0 | .001 |
| Not doing much physical activity | 49.7 | 56.1 | .002 | 55.1 | .001 |
| Infection with HPV (human papillomavirus) | 29.2 | 41.3 | .001 | 48.9 | .001 |
Barriers to Seeing a General Practitioner
Online survey participants were significantly more likely to endorse 8 of 14 barriers to seeing a GP than face-to-face participants. The most frequently endorsed barrier for face-to-face and agency B participants was “I find it difficult to get an appointment at a convenient time”; for agency A participants, it was “I don’t like having to talk to the GP receptionist.” Agency B participants were more likely than face-to-face participants to endorse an additional barrier “my doctor is difficult to talk to” (P=.001, OR 1.6, 95% CI 1.2-2.1). Figure 2 shows the percentage of participants that endorsed barriers to going to the doctor.
Figure 2.
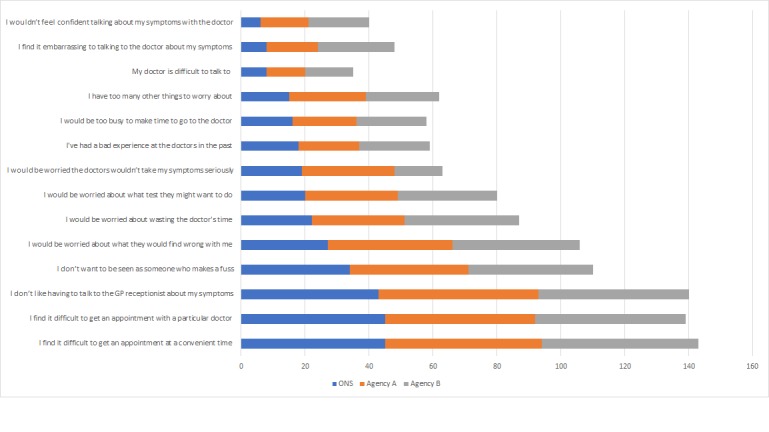
Percentage of participants that endorsed barriers to going to the doctor.
Interactions Between Outcomes and Demographic Variables.
Recall of bleeding or blood loss, cough, and difficulty swallowing showed significant interactions between sex and survey provider. For participants living in Scotland, those recruited by agency B were significantly less likely to recall bleeding or blood loss as a sign of cancer compared with those recruited by agency A (P=.04).
Fewer females recognized family history as a risk factor of cancer when completing face-to-face interviews than in online surveys (agency A females: P=.006; agency B females: P<.001). Significantly fewer males recognized not doing enough physical exercise as a risk factor of cancer in the agency B survey compared with agency A (P=.02).
Discussion
Analysis
This analysis explored the viability of moving from face-to-face to online data collection for the Cancer Research UK’s CAM.
Principal Results
Comparisons of data collected using face-to-face interviews and online surveys revealed minor differences between samples. Both methods provided broadly representative samples of the UK population with slight differences in awareness of signs, symptoms, and risk factors of cancer and frequency of help-seeking barriers reported, leading us to conclude that online data collection for the CAM is possible.
Recall of certain cancer signs and risk factors varied by demographic group. Recall of bleeding/blood loss, cough, and difficulty swallowing had significant interactions between sex and survey provider. Overall, recognition of risk factors was higher in the online surveys.
Recognition of risk factors varied by sex, education level, and country. Significantly fewer females recognized family history as a risk factor of cancer in the face-to-face survey compared with the online surveys. Significantly fewer males recognized not doing enough physical exercise as a risk factor of cancer in the online samples compared with the face-to-face sample. The reasons for these variations are unclear but provide avenues for further research and action.
Overall, online participants recruited by agency A were significantly more likely to recall cancer signs and risk factors compared with both agency B and face-to-face participants. This finding implies that agency A participants may be more engaged and knowledgeable than the other survey participants. Educational levels did not differ greatly among the three samples. Agency A participants may have been more engaged than other participants because they had previously taken part in a face-to-face survey, indicating that they may be a particularly motivated group.
Comparison With Prior Work
Previous research has found that levels of awareness of the HPV virus [26] and cholesterol [23] were higher among online than face-to-face or paper survey respondents. In this study, online participants recognized more risk factors than face-to-face participants, including being overweight, having a family history of cancer, eating too much red or processed meat, and infection with HPV (cholesterol was not assessed). However, only one of the online samples reported higher mean recall of risk factors compared with face-to-face participants. This particular panel, agency A, recruited participants after they had taken part in a paper survey, which may have resulted in a more engaged and knowledgeable sample.
Survey research within student populations has suggested that online participants are more likely to answer “don’t know” than those completing the same survey face-to-face [7]. Other research suggests that nonresponse to open-ended questions can be reduced through online data collection [8]. In this study, face-to-face participants were less likely than online participants to respond to recall questions around signs, symptoms, and risk factors with “don’t know.”
Socially desirable behaviors have been found to be less likely to be disclosed in interviews than online questionnaires [27,28]. In this study, online participants were more likely than face-to-face participants to endorse barriers to seeking help. Participants may have found it easier to endorse barriers to visiting the doctor with the context of anonymity afforded by online data collection compared with face-to-face data collection.
Strengths and Limitations
Although this study provides insights into the possibility of using online data collection for a large representative sample of the UK, there are limitations that warrant consideration. Regarding recruitment, large differences exist in the size of samples recruited online and face-to-face, highlighting the comparative ease of online recruitment. Previous research indicates that online research may not be as representative as face-to-face interviewing [29], but this is often based on the type of recruitment procedures that precede data collection. In this study, both online samples were recruited through panels; however, there may be differences in the ways that panels are recruited and incentivized, which may have affected the results. To mitigate this, each agency employed procedures to ensure their samples were as representative as possible of the Great Britain population.
For the analysis, it was not possible to calculate unique weighting variables, and we relied on those provided by agencies. The questions within each survey were identical; however, there may have been small differences in the presentation of questions within each sample.
It was necessary to limit the demographic variables studied to control the length of the survey, meaning that unobserved differences may have contributed to the differences observed.
It was not possible to compare the samples collected by each survey agency with the Great Britain population data. The Great Britain population data used were publicly available, although confidence intervals were not provided, and statistically significant comparisons were not possible.
It was not possible to access information about response rates or completion times within each sample. This information may have been useful to explore the differences among samples in more depth.
Conclusions
The relationships between sampling, sample representativeness, survey modality, and subsequent responses are complex. Although sample representativeness varied a little between samples and there are likely unobserved differences, we were encouraged to see that these variations were small overall. This information will be useful in helping us to tailor our recruitment strategy to ensure that we recruit a sample that is as representative as possible of the Great Britain population in future CAM research.
We observed larger differences when looking at responses to the awareness questions themselves, even between the two online samples, which point to the fact that there may be differences in the sampling and running of these panels contributing to these differences.
Nevertheless, the flexibility and potential cost savings of online data collection will enable larger samples and greater variation in content at a lower cost, which will enable the CAM to explore a new and wider range of issues related to the early diagnosis, prevention, and treatment of cancer.
Acknowledgments
Many thanks to the agencies involved in the collection of data for this research. This research was funded by Cancer Research UK.
Abbreviations
- CAM
Cancer Awareness Measure
- HPV
human papillomavirus
- NHS
National Health Service
- ONS
Office for National Statistics
Appendix
Survey Incentives and Survey Weighting Methodologies.
Number of cancer signs and risk factors recognised and recalled within each sample.
Footnotes
Conflicts of Interest: None declared.
References
- 1.World Health Organization. 2018. Sep 12, [2019-11-06]. Cancer 2018 https://www.who.int/news-room/fact-sheets/detail/cancer.
- 2.Cancer Research UK. 2018. [2019-11-06]. Cancer statistics for the UK https://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk#heading-One.
- 3.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R, EUROCARE Working Group EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009 Apr;45(6):931–991. doi: 10.1016/j.ejca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Verdecchia A, Guzzinati S, Francisci S, De Angelis R, Bray F, Allemani C, Tavilla A, Santaquilani M, Sant M, EUROCARE Working Group Survival trends in European cancer patients diagnosed from 1988 to 1999. Eur J Cancer. 2009 Apr;45(6):1042–1066. doi: 10.1016/j.ejca.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Maringe C, Walters S, Rachet B, Butler J, Fields T, Finan P, Maxwell R, Nedrebø B, Påhlman L, Sjövall A, Spigelman A, Engholm G, Gavin A, Gjerstorff ML, Hatcher J, Johannesen TB, Morris E, McGahan CE, Tracey E, Turner D, Richards MA, Coleman MP, ICBP Module 1 Working Group Stage at diagnosis and colorectal cancer survival in six high-income countries: a population-based study of patients diagnosed during 2000-2007. Acta Oncol. 2013 Jun;52(5):919–932. doi: 10.3109/0284186X.2013.764008. [DOI] [PubMed] [Google Scholar]
- 6.Møller H, Sandin F, Bray F, Klint A, Linklater KM, Purushotham A, Robinson D, Holmberg L. Breast cancer survival in England, Norway and Sweden: a population-based comparison. Int J Cancer. 2010 Dec 01;127(11):2630–2638. doi: 10.1002/ijc.25264. doi: 10.1002/ijc.25264. [DOI] [PubMed] [Google Scholar]
- 7.Walters S, Maringe C, Coleman MP, Peake MD, Butler J, Young N, Bergström S, Hanna L, Jakobsen E, Kölbeck K, Sundstrøm S, Engholm G, Gavin A, Gjerstorff ML, Hatcher J, Johannesen TB, Linklater KM, McGahan CE, Steward J, Tracey E, Turner D, Richards MA, Rachet B, ICBP Module 1 Working Group Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax. 2013 Jun;68(6):551–564. doi: 10.1136/thoraxjnl-2012-202297. [DOI] [PubMed] [Google Scholar]
- 8.Niksic M, Rachet B, Duffy SW, Quaresma M, Møller H, Forbes LJ. Is cancer survival associated with cancer symptom awareness and barriers to seeking medical help in England? An ecological study. Br J Cancer. 2016 Sep 27;115(7):876–886. doi: 10.1038/bjc.2016.246. doi: 10.1038/bjc.2016.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyratzopoulos G, Neal RD, Barbiere JM, Rubin GP, Abel GA. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 2012 Apr;13(4):353–365. doi: 10.1016/S1470-2045(12)70041-4. [DOI] [PubMed] [Google Scholar]
- 10.Bomb M, Hiom S, Kumar H, Moffat J, Ormiston-Smith N, Woolf L. Saving Lives, Averting Costs. London: Cancer Research UK; 2014. Sep, https://www.cancerresearchuk.org/sites/default/files/saving_lives_averting_costs.pdf. [Google Scholar]
- 11.McPhail S, Johnson S, Greenberg D, Peake M, Rous B. Stage at diagnosis and early mortality from cancer in England. Br J Cancer. 2015 Mar 3;112(S1):S108–S115. doi: 10.1038/bjc.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stubbings S, Robb K, Waller J, Ramirez A, Austoker J, Macleod U, Hiom S, Wardle J. Development of a measurement tool to assess public awareness of cancer. Br J Cancer. 2009 Dec 3;101(S2):S13–S17. doi: 10.1038/sj.bjc.6605385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osborne K. Cancer Research UK. 2016. [2019-11-06]. Cancer Awareness Measure (CAM) key findings report; 2014 & trends analysis (2008-2014) https://www.cancerresearchuk.org/health-professional/awareness-and-prevention/the-cancer-awareness-measures-cam.
- 14.Niksic M, Rachet B, Warburton FG, Wardle J, Ramirez AJ, Forbes LJL. Cancer symptom awareness and barriers to symptomatic presentation in England—are we clear on cancer? Br J Cancer. 2015 Jun 30;113(3):533–542. doi: 10.1038/bjc.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robb K, Stubbings S, Ramirez A, Macleod U, Austoker J, Waller J, Hiom S, Wardle J. Public awareness of cancer in Britain: a population-based survey of adults. Br J Cancer. 2009 Dec 03;101 Suppl 2:S18–S23. doi: 10.1038/sj.bjc.6605386. doi: 10.1038/sj.bjc.6605386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niksic M, Rachet B, Duffy SW, Quaresma M, Møller H, Forbes LJ. Is cancer survival associated with cancer symptom awareness and barriers to seeking medical help in England? An ecological study. Br J Cancer. 2016 Aug 18;115(7):876–886. doi: 10.1038/bjc.2016.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunswick N, Wardle J, Jarvis MJ. Public awareness of warning signs for cancer in Britain. Cancer Causes Control. 2001 Jan;12(1):33–37. doi: 10.1023/a:1008975416756. [DOI] [PubMed] [Google Scholar]
- 18.Marlow L, Robb K, Simon A, Waller J, Wardle J. Awareness of cancer risk factors among ethnic minority groups in England. Public Health. 2012 Aug;126(8):702–709. doi: 10.1016/j.puhe.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Office for National Statistics. 2018. Aug 07, [2018-08-07]. Internet Access: Households and Individuals in Great Britain https://www.ons.gov.uk/peoplepopulationandcommunity/householdcharacteristics/homeinternetandsocialmediausage/bulletins/internetaccesshouseholdsandindividuals/2018.
- 20.Kongsved SM, Basnov M, Holm-Christensen K, Hjollund NH. Response rate and completeness of questionnaires: a randomized study of internet versus paper-and-pencil versions. J Med Internet Res. 2007 Sep 30;9(3):e25. doi: 10.2196/jmir.9.3.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright K. Researching internet-based populations: advantages and disadvantages of online survey research, online questionnaire authoring software packages, and web survey services. J Comput-Mediat Comm. 2005;10(3) doi: 10.1111/j.1083-6101.2005.tb00259.x. [DOI] [Google Scholar]
- 22.Roster CA, Rogers RD, Albaum G, Klein D. A comparison of response characteristics from web and telephone surveys. Int J Market Res. 2018 Jan 30;46(3):359–373. doi: 10.1177/147078530404600301. [DOI] [Google Scholar]
- 23.Duffy B, Smith K, Terhanian G, Bremer J. Comparing data from online and face-to-face surveys. Int J Market Res. 2018 Jan 30;47(6):615–639. doi: 10.1177/147078530504700602. [DOI] [Google Scholar]
- 24.Comley P. Innovation in online research - who needs online panels?. MRS Research Conference - Paper 36; MRS Research Conference; 2003; London. 2003. [Google Scholar]
- 25.Taylor H, Krane D, Thomas RK. How does social desirability affect responses? Differences in telephone and online. The American Association for Public Opinion Research (AAPOR) 60th Annual Conference; May 12-15, 2005; Miami, FL. 2005. [DOI] [Google Scholar]
- 26.Sherman SM, Nailer E, Minshall C, Coombes R, Cooper J, Redman CW. Awareness and knowledge of HPV and cervical cancer in female students: a survey (with a cautionary note) J Obstet Gynaecol. 2016;36(1):76–80. doi: 10.3109/01443615.2015.1041886. [DOI] [PubMed] [Google Scholar]
- 27.Gnambs T, Kaspar K. Disclosure of sensitive behaviors across self-administered survey modes: a meta-analysis. Behav Res. 2014 Nov 20;47(4):1237–1259. doi: 10.3758/s13428-014-0533-4. [DOI] [PubMed] [Google Scholar]
- 28.Trau RN, Härtel CE, Härtel GF. Reaching and hearing the invisible: organizational research on invisible stigmatized groups via web surveys. Brit J Manage. 2012 Apr 19;24(4):532–541. doi: 10.1111/j.1467-8551.2012.00826.x. [DOI] [Google Scholar]
- 29.Szolnoki G, Hoffmann D. Online, face-to-face and telephone surveys—comparing different sampling methods in wine consumer research. Wine Econ Policy. 2013 Dec;2(2):57–66. doi: 10.1016/j.wep.2013.10.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survey Incentives and Survey Weighting Methodologies.
Number of cancer signs and risk factors recognised and recalled within each sample.


