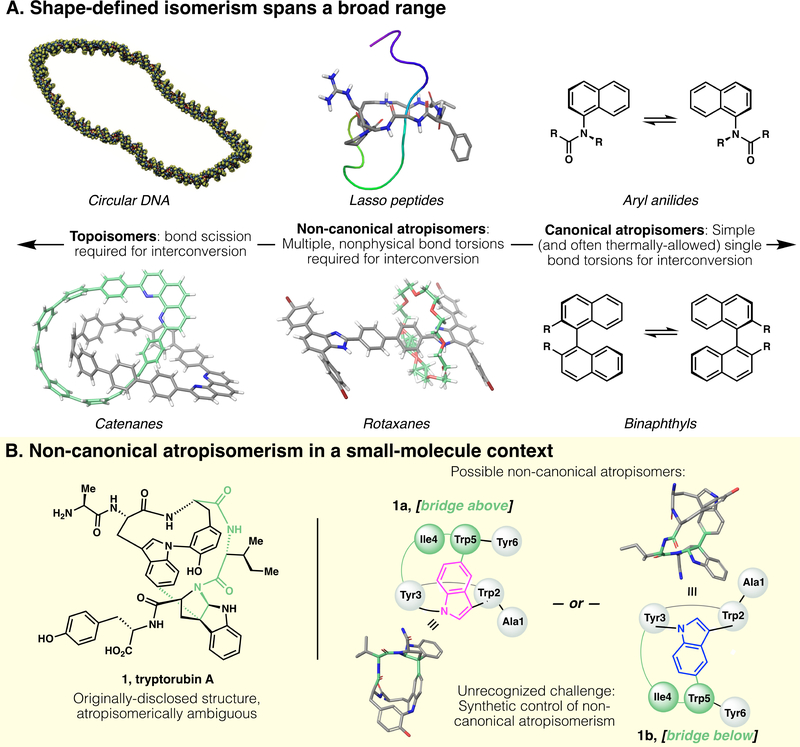
Fig. 1.
(A) Shape-based isomerism in synthetic and natural products spans a broad range. At one end (left), defined topology encodes topoisomers. At the other end (right) canonical atropisomerism is defined by simple axial differences (i.e., torsion of a single bond). Under the broad umbrella of atropisomerism, but distinct from more canonical examples, are (center) non-canonical atropisomers that are formally topologically trivial, but whose interconversion requires complex multibond rotations and unphysical torsions. Historically, this area has been occupied only by macromolecules; in this work, we disclose a small molecule natural product that presents this type of non-canonical atropisomerism. Structures obtained from PDB and/or CCDC database: circular DNA, reproduced from ref (4); lasso peptide, PDB 5TJ1 (5); catenane, CCDC #1835146 (6); rotaxane, CCDC #1576710 (7). (B) Left, originally-proposed structure of tryptorubin A; Right, two non-canonical atropisomers are possible within the limits of the originally-proposed 2D structure. Note: 3D structures of 1a and 1b are computed, not crystallographic, and their terminal residues are truncated for clarity.