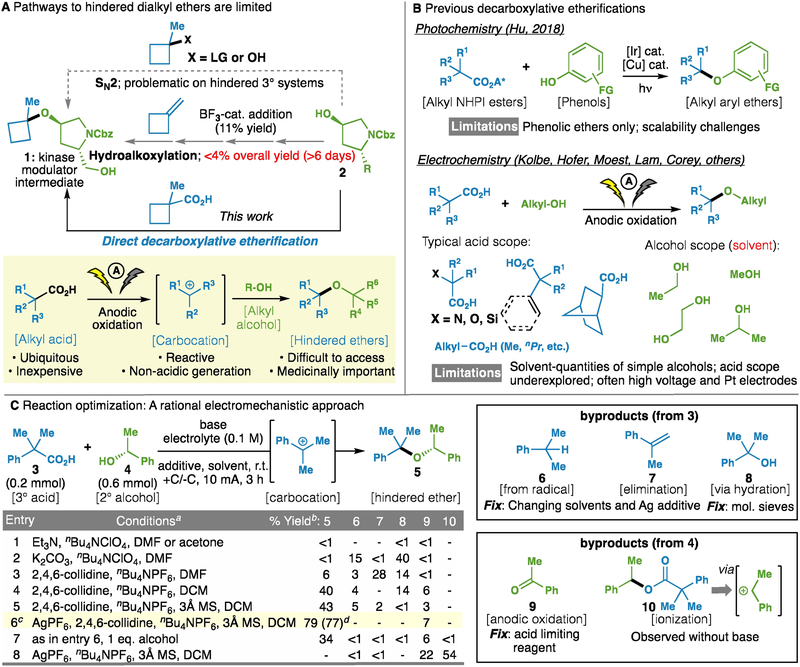
Figure 1.
(A) The synthesis of hindered ethers is a long-standing challenge in organic synthesis; (B) Historical context and prior strategies for decarboxylative etherification; and (C) Development and optimization of hindered ether synthesis depicted through electromechanistic analysis. aCompound 3 (0.2 mmol), 3.0 eq. alcohol 4 was used (except where designated). bGC yield. All entries performed in triplicate. cConditions: acid 3 (0.2 mmol), alcohol 4 (0.6 mmol), AgPF6 (0.3 mmol), 2,4,6-collidine (0.6 mmol), nBu4NPF6 (0.1 M), 3Å MS (150 mg), CH2Cl2 (3 mL), I = 10 mA, 3 h. dIsolated yield. DMF, N,N-dimethylformamide; DCM, dichloromethane; 3Å MS, 3Å molecular sieves.