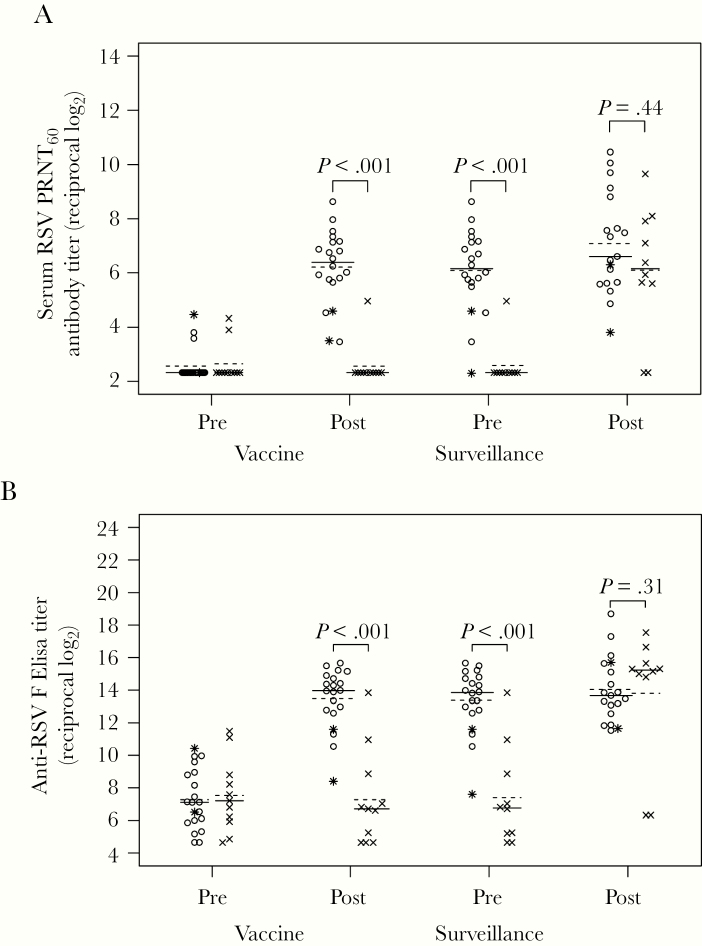
Figure 2.
Serum respiratory syncytial virus (RSV) antibody titers in vaccine and placebo recipients. Serum RSV 60% plaque reduction neutralizing antibody titers (PRNT60) (A) and anti-RSV F immunoglobulin (Ig)G enzyme-linked immunosorbent assay titers (ELISA) (B) were determined by complement-enhanced 60% plaque reduction neutralization assay and IgG-specific ELISA against purified RSV F protein, respectively, for vaccine (open circles) and placebo (x) recipients in sera collected at preinoculation (screening), postinoculation (study day 56), presurveillance (October 1–31), and postsurveillance (April 1–30, after the RSV season). Two vaccinees who did not shed vaccine virus and did not have a ≥4-fold increase in either antibody response are indicated by asterisks instead of open circles. The lines indicate median (solid line) and mean (dashed line) values. Serum antibody titers are expressed as the reciprocal log2. P values were determined by Wilcoxon rank-sum test. Postsurveillance data are missing for 1 vaccine recipient, and pre- and postsurveillance data are missing for 1 placebo recipient.