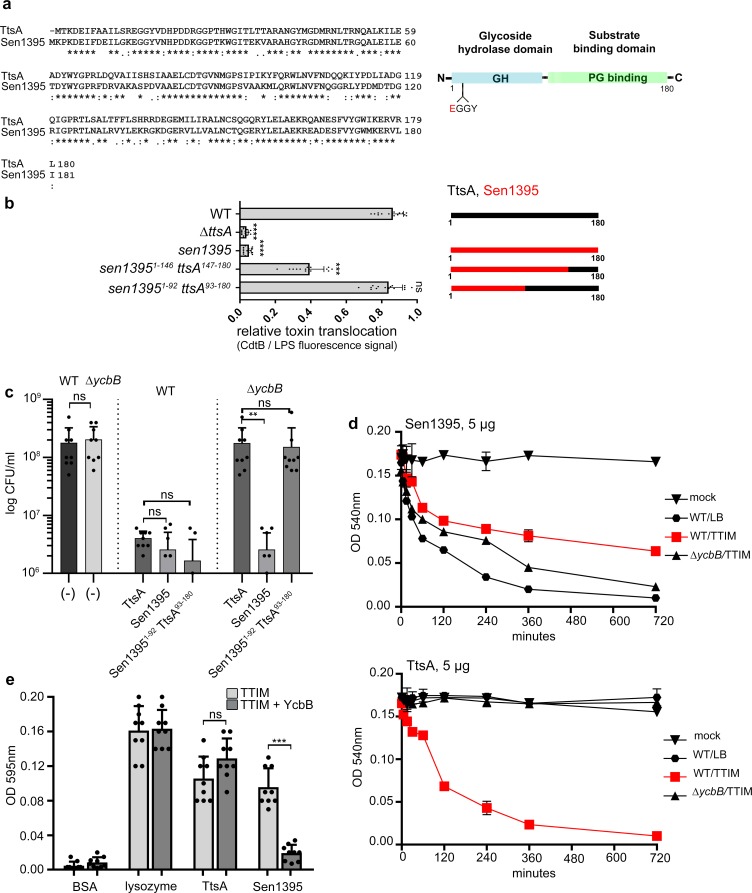
Figure 1. The carboxy-terminal domain of TtsA confers substrate specificity and typhoid toxin secretion functions.
(a) Amino acid sequence alignment between TtsA and its S. Enteritidis homolog Sen1395. Identical (*) and conserved (:) residues are indicated. The predicted domain organization is also shown. (b) Ability of S. Enteritidis Sen1395 and the indicated chimeras to complement a S. Typhi ∆ttsA mutant strain for typhoid toxin translocation across the PG. The relative amount of typhoid toxin translocation across the PG was quantified by immunofluorescence microscopy after staining with antibodies to the FLAG epitope (to visualize CdtB, a component of typhoid toxin), and LPS (to visualize bacterial cells). The average ratios of typhoid toxin- and LPS-associated fluorescence intensity ± standard deviation are shown (****p<0.0001, ***p<0.001, ns p=0.395, two-sided Student’s t Test) (Figure 1—source data 1). For each experiment a total of 10 images were collected from which 100 randomly selected bacteria per image were analyzed. A diagram of the different constructs is also shown. (c) Effect of expression of TtsA, Sen1395 or a TtsA-Sen1395 chimera on the growth of wild type or ∆ycbB S. Typhi, as indicated. Overnight grown bacteria were subcultured (1:50) in TTIM and grown to an OD600 of 0.3, at which point 0.3% arabinose was added to the bacterial cultures to induce the expression of the different muramidases, and subsequently incubated for 20 hr at 37°C. Colony forming units (CFUs) were determined by plating bacterial dilutions on LB agar plates. Values represent the mean + /- standard deviation (**p<0.01, ns p=0.6892, p=0.0115, p=0.0145, p=0.7505, two-sided Student`s t-Test, when compared to the values of the respective control strains, shown on the left bars). (d-e) TtsA and Sen1395 muramidase activity on YcbB-edited PG. Peptidoglycan was isolated from wild-type S. Typhi grown in either LB, or TTIM, or from the ∆ycbB S. Typhi mutant grown in TTIM, as indicated (d). The PG hydrolytic activity of purified Sen1395 and TtsA was evaluated using a turbidimetric assay. Graphs show the mean turbidity (measured at OD540 nm) ± standard deviation (d). Alternatively, PG was isolated from wild-type S. Typhi or from a wild-type S. Typhi strain carrying a plasmid over-expressing ycbB under the control of an arabinose-inducible promoter (e). Both strains were grown for 24 hr in TTIM containing 0.3% arabinose. The PG hydrolytic activity of purified Sen1395, TtsA, lysozyme (positive control) and BSA (negative control) was evaluated using a Remazol Brilliant Blue (RBB)-dye release assay. The dye bound to PG, is released to the supernatant due to PG hydrolysis and can be measured by its absorbance. Graphs show the mean absorbance (measured at OD595 nm)± standard deviation (***p<0.0026, ns p=0.6892, two-sided Student`s t-Test). (b–e) All data are derived from at least three independent experiments (Figure 1—source data 1).
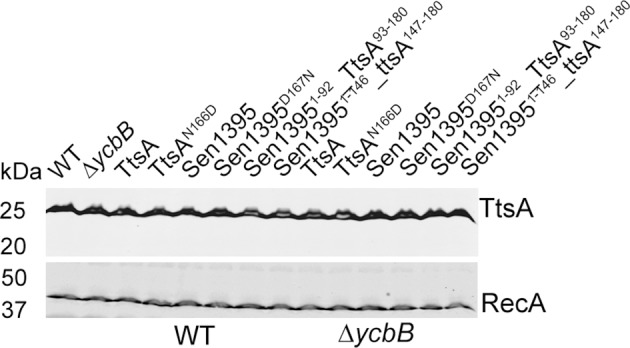
Figure 1—figure supplement 1. Western blot analysis of the expression levels of CdtB and TtsA in the indicated S.
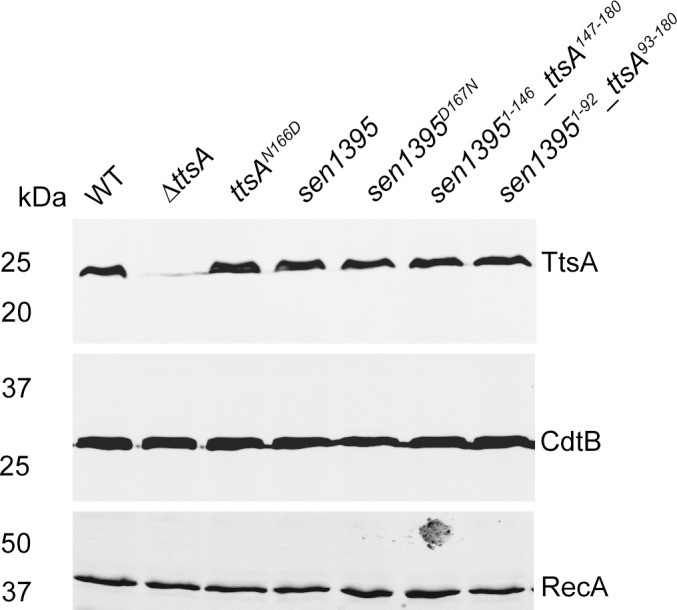
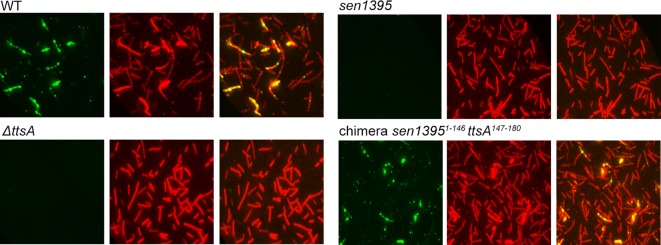
Figure 1—figure supplement 2. Ability of S. Enteritidis Sen1395 and the indicated chimera to complement a S. Typhi ∆ttsA mutant strain for typhoid toxin translocation across the PG.
Figure 1—figure supplement 3. Western blot analysis of the expression levels of the indicated plasmid-born FLAG-tagged TtsA, Sen1395 or chimeric proteins expressed from an arabinose-inducible promoter in the S. Typhi strains assayed in Figure 1C.