Figure 2. The carboxy-terminus of TtsA controls polar localization.
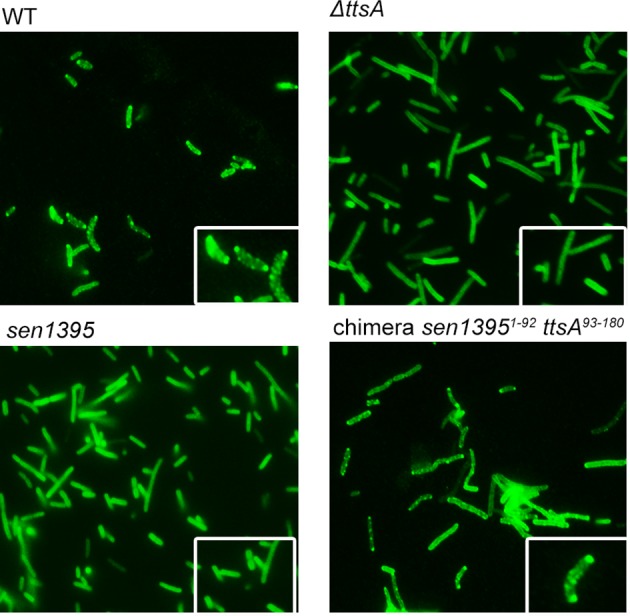
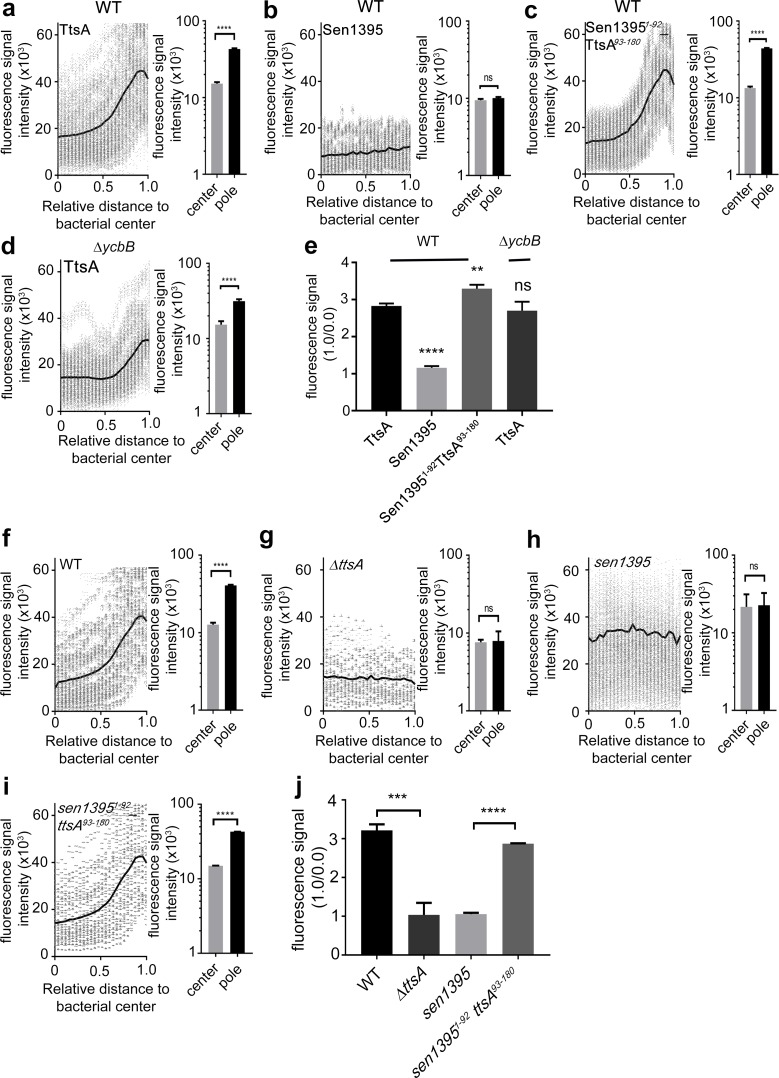
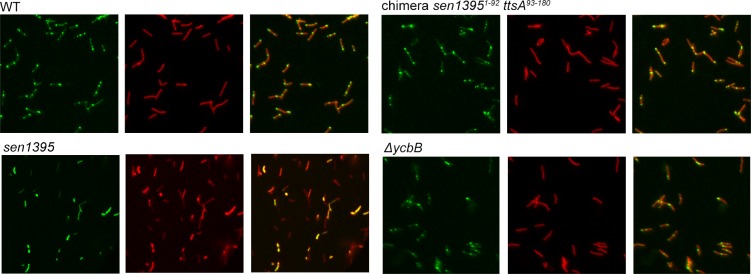
(a–e) Subcellular distribution of TtsA, Sen1395, and chimeric proteins. Wild type (a–c) or ∆ycbB (d) S. Typhi strains carrying chromosomally-encoded FLAG-tagged TtsA (a and d), Sen1395 (b) or the chimeric Sen1395-TtsA protein (c) were grown for 24 hr in TTIM, fixed, and stained with a mouse antibody directed to the 3xFLAG-epitope (green) (to visualize the muramidases) and a rabbit antibody directed to S. Typhi LPS (red). The scatter plots show fluorescence signal intensities for the indicated FLAG-tagged protein distributed along the axes of individual bacterial cells. Twenty-six measuring points were defined from the center (0) to each of the poles (1.0) of the bacterial cells and the distribution of the fluorescence intensity along the axes of bacteria were analyzed with the MicrobeJ plug-in of ImageJ (https://imagej.nih.gov/ij). The black line depicts the average of the intensities measured at each of the 26 measuring points. Data in each panel are from 1800 individual measurements at each of the measuring points from 900 bacteria analyzed in opposite directions from the center. The bar graphs next to each scatter plot show the quantification of the signal intensities at the measuring point furthest from the center (1.0), and at the center of each bacterium (0). Data represent the mean ± standard deviation from 1800 measurements (****p<0.0001, ns p=0.0864, two-sided Student`s t-Test) (a–d). (e) Bar graph shows the average ratios between the signal intensity of the indicated proteins measured at the furthest point from the center (1.0) and at the center of each bacterium (0.0) Data represent the mean ± standard deviation (****p<0.0001, **p<0.01, ns p=0.4369, two-sided Student`s t-Test). (f–j) TtsA- and Sen1395-mediated PG remodeling. S. Typhi wild type expressing ttsA (f), its isogenic ∆ttsA mutant (g), or S. Typhi strains expressing either sen1395 (h) or a sen1395-ttsA chimera (i) were grown in TTIM for 24 hr and the PG was metabolically labeled with alkyne-D-alanine. Remodeling PG was subsequently revealed with azido-AF488 after its linkage to the alkyne-D-alanine that had been incorporated into the PG layer. The scatter plots show the results of line scan analyses of fluorescence signal intensities along the axes of individual bacterial cells as described above. The line depicts the average fluorescence for each measured point. The bar graphs next to each scatter plot show the average ratios of the signal intensities measured at the furthest point from the center (1.0) and at the center of each bacterium (0.0). Data represent the mean ± standard deviation from 1800 measurements (****p<0.0001, ns p=0.8464 and p=0.8993, two-sided Student`s t-Test) (f–i). (j) Bar graph shows the average ratios between the signal intensity measured at the point furthest from the center (1.0) and at the center of each bacterium (0.0). Data represent the mean ± standard deviation (***p<0.001, ****p<0.0001, two-sided Student`s t-Test). (a–j) All data are derived from at least three independent experiments (Figure 2—source data 1).
Figure 2—figure supplement 1. Subcellular distribution of TtsA, Sen1395, and the indicated Sen1395-TtsA chimera.
Figure 2—figure supplement 2. TtsA- and Sen1395-mediated PG remodeling.