Abstract
Background
Diamond nanoparticles (Nanodiamond) are biocompatible drug delivery platforms with outstanding surface properties. Their passage into the brain has been confirmed previously. Thus, nanodiamond could provide a drug delivery system to shuttle several drugs through the blood-brain barrier (BBB) which represents a real challenge for the effective delivery of several drugs into the brain. Amlodipine is a calcium channel blocker that cannot pass through BBB and may elicit neuroprotective effects to reverse calcium-induced excitotoxicity and mitochondrial dysfunction that underlie several neurologic disorders including Alzheimer’s disease and stroke.
Aim
The study aimed to investigate the loading of amlodipine on nanodiamond particles.
Methods
Nanodiamond particles were oxidized in a strong oxidizing acidic mixture of sulfuric and nitric acids. Adsorption of amlodipine on nanodiamond particles was achieved in alkaline pH using various concentrations of sodium hydroxide. The loaded amlodipine was determined by high-performance liquid chromatography and confirmed by Fourier transform infrared (FTIR) spectroscopy and transmission electron microscopy.
Results
The highest percentage (41%) of loaded amlodipine onto nanodiamond particles was achieved in alkaline medium using 2 mM NaOH at a corresponding pH of 8.5. Also, characteristic FTIR bands of amlodipine and nanodiamond were shown obviously in the nanodiamond–amlodipine conjugates. Moreover, the successful loading of amlodipine on diamond nanoparticles was confirmed by transmission electron microscopy.
Conclusion
The present study demonstrates the successful loading of amlodipine onto nanodiamond particles. These findings offer a potential for applying diamond nanoparticles as a drug delivery system to shuttle amlodipine into the brain and open the door to deliver other similar drugs into the brain.
Keywords: diamond nanoparticles, nanodiamond, amlodipine, calcium channel antagonists, drug delivery system, blood-brain barrier, excitotoxicity
Introduction
Blood-brain barrier (BBB) represents a mechanical barrier and a dynamic biological entity that prevents the passage of most circulating cells and molecules into the brain.1,2 The BBB is formed by endothelial cells in the cerebral blood vessels, astrocytes, pericytes, and perivascular mast cells.3 The BBB is the single most important factor limiting the future growth of neurotherapeutics.4 The BBB prevents the brain uptake of most drugs, with the exception of small hydrophilic compounds with a mass lower than 150 Da and highly lipophilic compounds with a mass lower than 400–600 Da that can pass the membrane by passive diffusion.5 Various nanoparticles within the size of 1–100 nm were reported to cross the BBB.6 Diamond nanoparticles (nanodiamond) are an emerging important class of drug delivery platforms with extraordinary biological properties, structural characteristics, and unique surface properties that could be useful as drug delivery vehicles for delivering drugs into the brain. These nanoparticles are enriched with several functional groups that could adsorb or conjugate with several drugs, proteins, and nucleic acids. Nanodiamond-mediated adsorption of doxorubicin is a promising example for the application of nanodiamond particles as drug delivery platforms.7 Nanodiamond particles have an adamantane nucleus in their structures, which present in several neuroprotective drugs including memantine (used in the treatment of Alzheimer’s disease), amantadine (used in the treatment of Parkinson’s disease). Nanodiamond has been demonstrated to undergo systemic distribution and exhibits neuroprotective effects.8
Alzheimer’s disease represents an increasingly important worldwide public health problem; it is the principal cause of dementia throughout the world9 and the fourth cause of death in developed countries after cancer, cardiovascular diseases, and vascular stroke.10 Alzheimer’s disease is associated with irreversible progressive neurodegenerative pathological changes that impair memory and learning due to apoptosis of selective neuronal populations predominantly in the hippocampus and cerebral cortex. The main pathologic hallmarks of Alzheimer’s disease are accumulation of extracellular beta-amyloid (Aβ) senile plaques and intracellular neurofibrillary tangles which are formed due to abnormal hyperphosphorylation of cytoskeletal tau protein.11 Aβ oligomers are incorporated into neuronal membranes resulting in the formation of ion channels with subsequent Ca2+ influx through these amyloid channels.12 Excessive calcium influx into the cell plays an important role in phosphorylation of tau, formation of free radicals, and depletion of neurotrophic factors with the outcome of neuronal death.13
Calcium influx leads to complex I (NADH: ubiquinone oxidoreductase) inhibition, blocking the electron flow along the mitochondrial respiratory chain and increased reactive oxygen species production with subsequent release of pro-apoptotic factors like cytochrome C from the mitochondrial intermembrane space into the cytosol leading to caspase activation and eventually apoptosis.14–16 Calcium channel blockade attenuates Aβ-induced neuronal decline in vitro and is neuroprotective in animal models,17 and Nimodipine which passes the BBB has neuroprotective effects in Alzheimer’s disease.18 Moreover, Memantine, an antagonist of the NMDA-Ca2+ channel receptor, has beneficial effects in Alzheimer’s disease patients.19 Thus, treatments that interrupt aberrant Ca2+ influx may be effective in blocking abnormal neuronal apoptosis and may be promising therapeutic strategies for Alzheimer’s disease.
Amlodipine is a dihydropyridine calcium channel blocker that cannot pass the BBB and may elicit a promising role to reverse calcium-induced excitotoxic neuronal cell death that underlies several neurodegenerative diseases including Alzheimer’s disease when delivered into the brain through the blood-brain barrier. Taking advantage of the unique surface properties of nanodiamond particles, the current study aimed to investigate the ability of amlodipine to be loaded on nanodiamond particles. The loading of amlodipine on nanodiamond particles was determined by High-Performance Liquid Chromatography (HPLC) and confirmed by Fourier Transform Infrared (FTIR) Spectroscopy and Transmission Electron Microscopy.
Materials and Methods
Chemicals and Drugs
Amlodipine maleate was purchased from Sigma-Aldrich (St. Louis, MO, USA), and nanodiamond powder with a particle size of about 20 nm (SYP GAF002) was purchased from Van Moppes (Geneva, Switzerland). Analytical HPLC grade Methanol (Sigma-Aldrich, USA), acetonitrile (Sigma-Aldrich, USA), triethylamine (BDH, UK), phosphoric acid (Merck, Germany), potassium bromide (Fluka, Switzerland), sodium hydroxide (Scharlau, Spain) were used.
Preparation of Nanodiamond Particles
The nanodiamond powder was oxidized/carboxylated in strong oxidizing conditions by treatment with a concentrated acid mixture of 68% HNO3: 98% H2SO4 (1:9 v/v) at 100°C for 48 h. Neutralization and washing were performed through several cycles of centrifugations (10,000 rpm for 5 mins) in deionized water and ultrasonic redispersion of the pellet. The resultant nanodiamond particles were suspended in deionized water, a stable colloidal dispersion was formed (after 3 months, neither sedimentation nor aggregation was observed). Size distribution was measured by dynamic light scattering at a scattering angle of 165° using a Delsa Nano C Particle Size apparatus (Beckman Coulter, Brea, CA, USA). Scattering data were collected for 70 individual measurements at a constant scattering angle and averaged for each sample. The distribution number determined for the treated nanodiamond particles was 15±5nm.
Nanodiamond–Amlodipine Loading and Optimization
Nanodiamond particles and amlodipine maleate were mixed at 5:2 ratios (w/w), in alkaline solutions using 1, 1.5, 2, 2.5, 3 mM NaOH. The alkaline media were aimed to promote the loading efficiency of amlodipine by deprotonating the carboxylic functional groups on nanodiamond particles. After incubation for 5 mins at 25°C, the solution was centrifuged at 2500 rpm for 15 mins to pellet bound nanodiamond–amlodipine and unbound amlodipine in the remained supernatant. The nanodiamond–amlodipine pellet was rinsed with water and dried under vacuum.
Nanodiamond–Amlodipine Characterization
The loading of amlodipine onto nanodiamond particles was determined by measuring the concentration of the remaining unbound amlodipine in the supernatants by HPLC as described in the United States Pharmacopoeia (USP),20 and the obtained values were used to calculate the amounts of bound amlodipine on nanodiamond particles in the pellets as nanodiamond–amlodipine conjugates. The pellets were subsequently subjected to further characterization by FTIR spectroscopy to identify the peaks of nanodiamond–amlodipine spectra and to verify the successful loading of amlodipine on nanodiamond particles. All experiments were performed in the National Quality Control Laboratory of the Supreme Board of Drugs and Medical Appliances, Sana’a, Yemen. Moreover, nanodiamond–amlodipine conjugates were examined under Transmission Electron Microscopy in the laboratories of the National Research Center, Cairo, Egypt.
Determination of Amlodipine in the Supernatant by HPLC
The pH 3.0 buffer was prepared by dissolving 7-mL triethylamine in 800 mL of water and the pH was adjusted to 3.0 ± 0.1 with phosphoric acid and diluted with water to 1 L. The mobile phase consisting of pH 3.0 buffer, methanol and acetonitrile mixture at a ratio of (50:35:15) was freshly prepared, filtered, degassed before use. The standard solution of amlodipine was prepared by dissolving 50.0-mg amlodipine maleate in the mobile phase to obtain a solution having a known concentration of 0.05-mg per mL. The assay solution of amlodipine was prepared by dissolving an accurately measured volume of the supernatant in the mobile phase to obtain a solution having a known amlodipine maleate concentration of about 0.05 mg/mL.
Apparatus and Chromatographic System
The chromatographic separation was performed on a Jasco LC-Net II/ADC chromatographic system (Jasco, Japan). The liquid chromatograph was equipped with a 237-nm detector and a 3.9-mm × 15-cm column containing a packing L1. The flow rate of the mobile phase was adjusted to 1 mL/minute. The volume of each injection of the standard and the assay was 10 μL. The column and the HPLC system were kept in ambient temperature. The standard preparation was injected into the loop and the chromatogram was recorded; the standard deviation for replicate injections was not more than 2%. The sample solution was injected under the same chromatographic conditions and the chromatogram was recorded. The percentage of amlodipine maleate in the supernatant of was taken by the formula: 100 (ru/rs) (Cs/Cu) where ru is the peak response of amlodipine in the assay preparation, rs is peak response of amlodipine in the standard preparation, Cs is the concentration of amlodipine in the standard preparation, and Cu is the concentration of amlodipine in the assay preparation. HPLC peaks were recorded for five injections of each sample of the assay (the supernatant) and standard (standard reference of amlodipine); the area of the average peaks was taken ± standard deviation.
Infrared Spectroscopy
Infrared (IR) spectra of nanodiamond particles, amlodipine, and nanodiamond–amlodipine conjugates samples were recorded on Shimadzu IR Tracer-100 Fourier Transform Infrared Spectrophotometer (Shimadzu, Japan) in the mid-IR range using a liquid nitrogen–cooled mercury cadmium telluride detector. All sample measurements were carried out in the solid state using pelletized, homogeneous powder dispersions of the samples in a potassium bromide matrix.
Results
HPLC Quantification
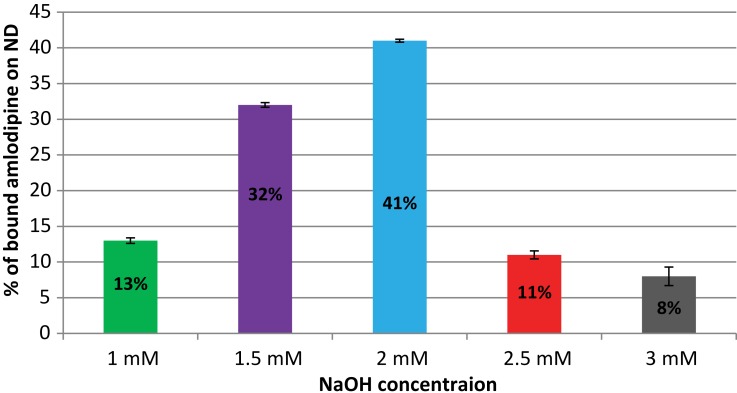
HPLC measurements showed variable loading of amlodipine onto diamond nanoparticles at different NaOH concentrations which were mounted 13 ±0.39, 32 ±0.33, 41 ±0.21, 11 ±0.56, and 8 ±1.30% (average mean ± SD) as the binding conditions were optimized using 1, 1.5, 2, 2.5, and 3 mM NaOH, respectively (Figure 1). The maximal loading of amlodipine onto nanodiamond was about 41% which was performed in 2 mM NaOH; this loaded amount is almost threefolds more as the loading in 1 mM NaOH (13%) and fivefolds as the loading in 3 mM NaOH (8%). Choosing the most effective binding condition showed that 2 mM of NaOH corresponding to a pH of 8.5 represented the highest percentage of amlodipine bound.
Figure 1.
Loading efficiency of amlodipine on nanodiamond at various NaOH concentrations various binding conditions (average mean ± SD).
FTIR – Characterization and Transmission Electron Microscopy
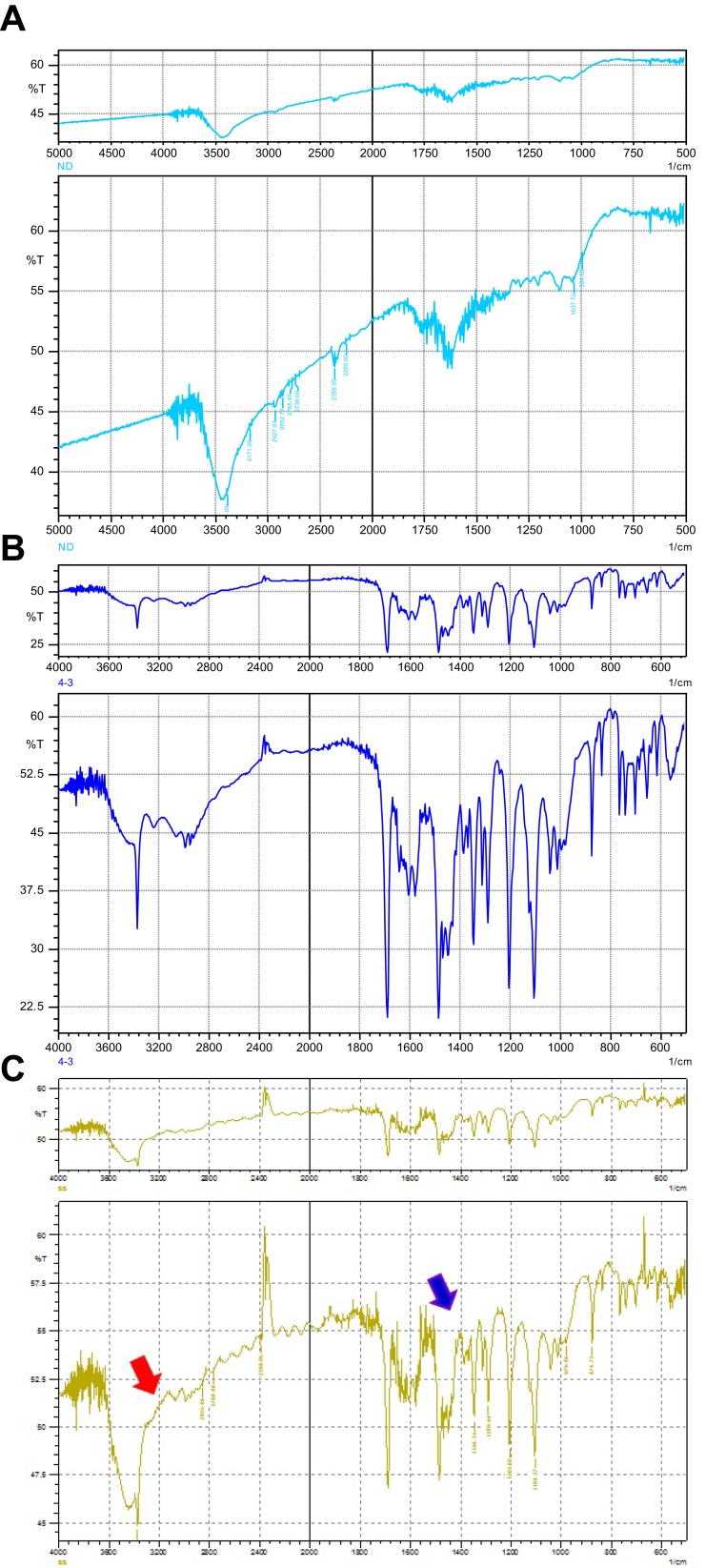
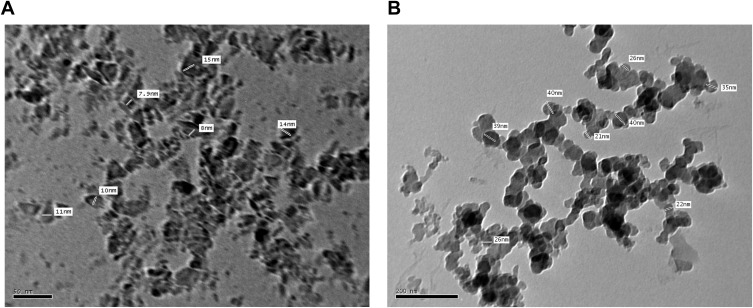
Nanodiamond surface analysis determined that the dominant functional group on nanodiamond is primarily carboxylic acid groups.21,22 Consistent with this finding, the nanodiamond spectra contained peaks for the C=O stretch and O-H bend signals, the primary structures found in the carboxylic acid group (Figure 2A). Examining the amlodipine spectra, there were strong signals for peaks corresponding to the N-H stretch and C-O-C stretch (Figure 2B) which indicate the presence of these groups in the amlodipine molecules, and not present in the nanodiamond spectra. Confirming the successful adsorption of amlodipine on nanodiamond platforms, characteristic bands seen in the FTIR spectra of nanodiamond and those unique to amlodipine were both well appeared in the FTIR spectra of nanodiamond–amlodipine conjugates (Figure 2C). These results were also confirmed by the micrographs of the Transmission Electron Microscopy of either nanodiamond particles (Figure 3A) or nanodiamond–amlodipine conjugates (Figure 3B). These micrographs showed that increased particle size of nanodiamond–amlodipine conjugates (31.1 ± 8.2 nm) compared with that of bare nanodiamond particles (11 ± 3 nm), confirming successful loading of amlodipine on nanodiamond particles.
Figure 2.
FTIR spectra depicting the characteristic peaks of the functional groups on nanodiamond particles and amlodipine: (A) FTIR spectra of nanodiamond particles; (B) FTIR spectra of amlodipine; (C) FTIR spectra of nanodiamond–amlodipine conjugates (strong peaks of amlodipine overlapped with nanodiamond particles spectra). Red arrow denotes to the FTIR peaks of nanodiamond particles; blue arrow denotes to the FTIR peaks of amlodipine.
Figure 3.
(A). Transmission electron microscopy micrograph of bare nanodiamond particles showing their average particle size of 11 ± 3 nm (average mean ± SD). (B). Transmission electron microscopy micrograph of nanodiamond–amlodipine conjugates showing increased particle size of nanodiamond–amlodipine conjugates (31.1 ± 8.2 nm) as compared with that of bare nanodiamond particles (average mean ± SD).
Discussion
Amlodipine is a dihydropyridine calcium channel blocker that cannot pass the blood-brain barrier and might elicit a promising role to reverse calcium-induced excitotoxic neuronal cell death that underlies many neurodegenerative diseases including Alzheimer’s disease when delivered into the brain. It has been demonstrated that calcium channel blockers that pass the blood-brain barrier like nimodipine have neuroprotective effects, and down-regulate expression of TNF-α and IL-1β in the hippocampus.23,24
Nanodiamond has demonstrated outstanding biocompatibility and facile surface functional groups as requisites of effective delivery vehicles.25–27 There are many practical requirements to drug delivery for platforms to retain a tight size distribution within the range of 10–150 nm.28,29 Smaller particles under 10 nm are eliminated by the kidney through renal excretion and larger particles are cleared by the mononuclear phagocytic system cells. Nanodiamond particles exhibit many crucial properties required of successful delivery agents, such as biocompatibility, narrow size distribution centered less than 100 nm in diameter, and readily reproducible and scalable processing techniques. The surfaces of nanodiamond particles are highly charged and heavily functionalized by carboxylic acid groups.21,22 These charged facets promote polar interactions with other highly charged groups and water molecules. Nanodiamond particles were treated by strong acidic treatment to expose the carboxylic functional groups on their surfaces, thereby forming a delivery system capable of shuttling amlodipine via electrostatic interaction with the amine group on amlodipine.
In this study, amlodipine has been loaded onto nanodiamond particles. It was bound probably by utilizing the surface electrostatic properties of nanodiamond. Conditions during nanodiamond synthesis and treatment result in a functionalized carbon surface of hydroxyl and carboxyl groups which can lead to a characteristic surface charge in aqueous solutions.30–32 At basic conditions, these groups become deprotonated and might interact with protonated amine group on the amlodipine molecule. Sodium hydroxide surface treatment deprotonated carboxylic acid groups on nanodiamond mediating substantial amlodipine loading. The adsorption mechanism for loading amlodipine is a quick and simple process that leads to a reversibly bound drug conjugate by electrostatic charge–charge interactions. Additionally, hydrogen bonds can form between –NH3+ and –COO− or other CO-containing surface groups, with H-bond binding energies between 10 and 30 Kcal/mol.33 –35
High-performance liquid chromatography measurements showed a variable decrease in amlodipine concentration in the supernatants at different NaOH concentrations. The maximal decrease in amlodipine concentration in the supernatant was about 41% with 2 mM NaOH, almost 3 times more as the loading at 1 mM NaOH and 5 times as the loading at 3 mM NaOH which mounted 13% and 8%, respectively. Notably, alkaline pH values promoted nanodiamond–amlodipine binding; however, higher pH values resulted in a substantial decrease in drug concentration in the supernatant. The later might be due to degradation of amlodipine at extreme pH, and this could explain the appearance of extra small peaks in the HPLC chromatogram rather than those for amlodipine as well as reduction of the peak area of amlodipine; these results are consistent with previous findings.36 Hence, the most effective binding of amlodipine onto nanodiamond was performed in 2 mM NaOH.
Fourier transform infrared (FTIR) spectroscopy confirmed the loading of amlodipine onto nanodiamond particles. Surface analysis has determined that the dominant functional group on nanodiamond is primarily carboxylic acid groups.21,22 Consistent with these findings, the FTIR spectra of nanodiamond in the present study contained peaks for the C=O stretch and O-H bend signals of the carboxylic acid groups. Similarly, amlodipine spectra displayed strong signals corresponding to the functional groups on the amlodipine molecule. Confirming the successful adsorption of amlodipine on nanodiamond, characteristic bands seen in the FTIR spectra of nanodiamond and even those unique to amlodipine were both obviously appeared in the FTIR spectra of nanodiamond–amlodipine conjugates.
Conclusion
For the first time, we have demonstrated the adsorption of amlodipine onto nanodiamond particles which have been previously proved to pass into the brain. The loading was determined by measuring amlodipine concentration in the supernatant by HPLC assay and verified by the appearance of the characteristic bands of both amlodipine and nanodiamond in the FTIR spectra of nanodiamond–amlodipine conjugates. Moreover, the loading of amlodipine on nanodiamond particles was confirmed in the micrographs of transmission electron microscopy. These findings offer the potential of applying nanodiamond–amlodipine as a drug delivery system to shuttle amlodipine into the brain and may open the door to deliver other similar drugs into the brain.
Acknowledgments
The authors gratefully acknowledge staff cooperation in the National Quality Control Laboratory of the Supreme Board of Drugs and Medical Appliances, Sana’a, Yemen, during HPLC and FTIR spectroscopy measurements – in particular, Dr. Ali Abdu Saif, Dr. Fadhl Jaadan, and Dr. Ibtesam Al-Fadhli. The authors would also like to express their sincere thanks to Dr. Marie-Odile David, Laboratory of Structure and Activity of Normal and Pathological Biomolecules, National Institute of Health and Medical Research, Evry-Val d'Essonne University, France for providing the diamond nanoparticles (nanodiamond) and her efforts during preparatio and chemical processing of the sample.
Abbreviations
Aβ, Amyloid beta; BBB, Blood-brain barrier; Da, Dalton (the atomic mass unit); FTIR, Fourier transform infrared spectroscopy; HPLC, High-performance liquid chromatography; NADH, Nicotinamide adenine dinucleotide – the reduced form; ND, nanodiamond; NMDA, N-methyl-D-aspartate; rcf, relative centrifugal force; rpm, revolutions per minute; ROS, Reactive oxygen species; USP, The United States Pharmacopeia.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
- 1.Loscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005;76(1):22–76. [DOI] [PubMed] [Google Scholar]
- 2.Petty MA, Lo EH. Junctional complexes of the blood brain barrier: permeability changes in neuroinflammation. Prog Neurobiol. 2002;68(5):311–323. doi: 10.1016/S0301-0082(02)00128-4 [DOI] [PubMed] [Google Scholar]
- 3.Wahl M, Unterberg A, Baethmann A, Schilling L. Mediators of blood-brain barrier dysfunction and formation of vasogenic brain edema. J Cereb Blood Flow Metab. 1988;8(5):621–634. doi: 10.1038/jcbfm.1988.109 [DOI] [PubMed] [Google Scholar]
- 4.Pardridge WM. Brain Drug Targeting: The Future of Brain Drug Development. Cambridge (UK): Cambridge University Press; 2001. [Google Scholar]
- 5.Santaguida S, Janigro D, Hossain M, Oby E, Rapp E, Cucullo L. Side by side comparison between dynamic versus static models of blood-brain barrier in vitro: a permeability study. Brain Res. 2006;1109(1):1–13. [DOI] [PubMed] [Google Scholar]
- 6.Sharma HS, Hussain S, Schlager J, Ali SF, Sharma A. Influence of nanoparticles on blood-brain barrier permeability and brain edema formation in rats. Acta Neurochir Suppl. 2010;106:359–364. [DOI] [PubMed] [Google Scholar]
- 7.Chow EK, Zhang XQ, Chen M, et al. Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci Transl Med. 2011;3(73):73ra21. doi: 10.1126/scitranslmed.3001713 [DOI] [PubMed] [Google Scholar]
- 8.Alawdi SH, El-Denshary ES, Safar MM, Eidi H, David MO, Abdel-Wahhab MA. Neuroprotective effect of nanodiamoND in Alzheimer’s disease rat model: a pivotal role for modulating NF-κB and STAT3 signaling. Mol Neurobiol. 2017;54(3):1906–1918. doi: 10.1007/s12035-016-9762-0 [DOI] [PubMed] [Google Scholar]
- 9.Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Eng J Med. 2003;348(14):1356–1364. doi: 10.1056/NEJM2003ra020003 [DOI] [PubMed] [Google Scholar]
- 10.Jovanović Z. Mechanisms of neurodegeneration in Alzheimer’s disease. Med Pregl. 2012;65(7–8):301–307. doi: 10.2298/MPNS1208301J [DOI] [PubMed] [Google Scholar]
- 11.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741 [DOI] [PubMed] [Google Scholar]
- 12.Kawahara M. Neurotoxicity of β-amyloid protein: oligomerization, channel formation, and calcium dyshomeostasis. Curr Pharm Des. 2010;16(25):2779–2789. doi: 10.2174/138161210793176545 [DOI] [PubMed] [Google Scholar]
- 13.Kawahara M, Kato-Negishi M. Link between aluminum and the pathogenesis of Alzheimer’s disease: the integration of the aluminum and amyloid cascade hypotheses. Int J Alzheimers Dis. 2011;2011:276393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizzuto R, Marchi S, Bonora M, et al. Ca2+ transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta. 2009;1787:1342–1351. doi: 10.1016/j.bbabio.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decuypere JP, Monaco G, Bultynck G, Missiaen L, De Smedt H, Parys JB. The IP3 receptor-mitochondria connection in apoptosis and autophagy. Biochimica Et Acta. 2011;1813:1003–1013. doi: 10.1016/j.bbamcr.2010.11.023 [DOI] [PubMed] [Google Scholar]
- 16.Chomova M, Racay P. Mitochondrial complex I in the network of known and unknown facts. Gen Physiol Biophys. 2010;29:3–11. doi: 10.4149/gpb_2010_01_3 [DOI] [PubMed] [Google Scholar]
- 17.Nimmrich V, Eckert A. Calcium channel blockers and dementia. Br J Pharmacol. 2013;169(6):1203–1210. doi: 10.1111/bph.12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Deng Y, Zhang X, Wang T, Wu F. Development and pharmacokinetics of nimodipine-loaded liposomes. J Pharm Pharmacol. 2006;58:1289–1294. doi: 10.1211/jpp.58.9.0017 [DOI] [PubMed] [Google Scholar]
- 19.Chen H-SV, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x [DOI] [PubMed] [Google Scholar]
- 20.United States Pharmacopeia and National Formulary (USP 30-NF 25). Vol 2 Rockville (MD): United States Pharmacopeia Convention; 2007:1400–1401. [Google Scholar]
- 21.Mohan N, Chen CS, Hsieh HH, Wu YC, Chang HC. In vivo imaging and toxicity assessments of fluorescent nanodiamonds in caenorhabditis elegans. Nano Lett. 2010;10:3692–3699. doi: 10.1021/nl1021909 [DOI] [PubMed] [Google Scholar]
- 22.Yuan Y, Chen Y, Lui J-H, Wang H, Liu Y. Biodistribution and fate of nanodiamonds in vivo. Diamond Relat Mater. 2009;18:95–100. doi: 10.1016/j.diamond.2008.10.031 [DOI] [Google Scholar]
- 23.Hashioka S, Klegeris A, McGeer PL. Inhibition of human astrocyte and microglia neurotoxicity by calcium channel blockers. Neuropharmacology. 2012;63:685–691. doi: 10.1016/j.neuropharm.2012.05.033 [DOI] [PubMed] [Google Scholar]
- 24.Zhang XL, Zheng SL, Dong FR, Wang ZM. Nimodipine improves regional cerebral blood flow and suppresses inflammatory factors in the hippocampus of rats with vascular dementia. J Int Med Res. 2012;40:1036–1045. doi: 10.1177/147323001204000322 [DOI] [PubMed] [Google Scholar]
- 25.Schrand AM, Huang H, Carlson C, et al. Are diamond nanoparticles cytotoxic? J Phys Chem B. 2006;111(1):2–7. doi: 10.1021/jp066387v [DOI] [PubMed] [Google Scholar]
- 26.Osawa E, Ho D, Huang H, Korobov MV, Rozhkova NN. Consequences of strong and diverse electrostatic potential fields on the surface of detonation nanodiamond particles. Diamond Relat Mater. 2009;18:904–909. doi: 10.1016/j.diamond.2009.01.025 [DOI] [Google Scholar]
- 27.Schmidlin L, Pichot V, Comet M, Josset S, Rabu P, Spitzer D. Identification, quantification and modification of detonation nanodiamond functional groups. Diamond Relat Mater. 2012;22:113–117. doi: 10.1016/j.diamond.2011.12.009 [DOI] [Google Scholar]
- 28.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614 [DOI] [PubMed] [Google Scholar]
- 29.Gaumet M, Vargas A, Gurny R, Delie F. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur J Pharm Biopharm. 2008;69:1–9. doi: 10.1016/j.ejpb.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 30.Huang H, Pierstorff E, Osawa E, Ho D. Active nanodiamond hydrogels for chemotherapeutic delivery. Nano Lett. 2007;7:3305–3314. doi: 10.1021/nl071521o [DOI] [PubMed] [Google Scholar]
- 31.Huang H, Pierstorff E, Osawa E, Ho D. Protein-mediated assembly of nanodiamond hydrogels into a biocompatible and biofunctional multilayer nanofilm. ACS Nano. 2008;2:203–212. doi: 10.1021/nn7000867 [DOI] [PubMed] [Google Scholar]
- 32.Yeap WS, Chen S, Loh KP. Detonation nanodiamond: an organic platform for the suzuki coupling of organic molecules. Langmuir. 2009;25:185–191. doi: 10.1021/la8029787 [DOI] [PubMed] [Google Scholar]
- 33.Zhao W, Xu JJ, Qiu QQ, Chen HY. Nanocrystalline diamond modified gold electrode for glucose biosensing. Biosens Bioelectron. 2006;22:649–655. doi: 10.1016/j.bios.2006.01.026 [DOI] [PubMed] [Google Scholar]
- 34.Huang LCL, Chang HC. Adsorption and immobilization of cytochrome c on nanodiamonds. Langmuir. 2004;20:5879–5884. doi: 10.1021/la0495736 [DOI] [PubMed] [Google Scholar]
- 35.Speller CV, Meot-Ner M. The ionic hydrogen bond and ion solvation. 3. Bonds involving cyanides. Correlations with proton affinities. J Phys Chem. 1985;89:5217–5222. doi: 10.1021/j100270a020 [DOI] [Google Scholar]
- 36.Jakimska A, Sliwka-Kaszynska M, Nagorski P, Namiesnik J, Kot-Wasik A. Phototransformation of amlodipine: degradation kinetics and identification of its photoproducts. PLoS One. 2014;9(10):e109206. doi: 10.1371/journal.pone.0109206 [DOI] [PMC free article] [PubMed] [Google Scholar]