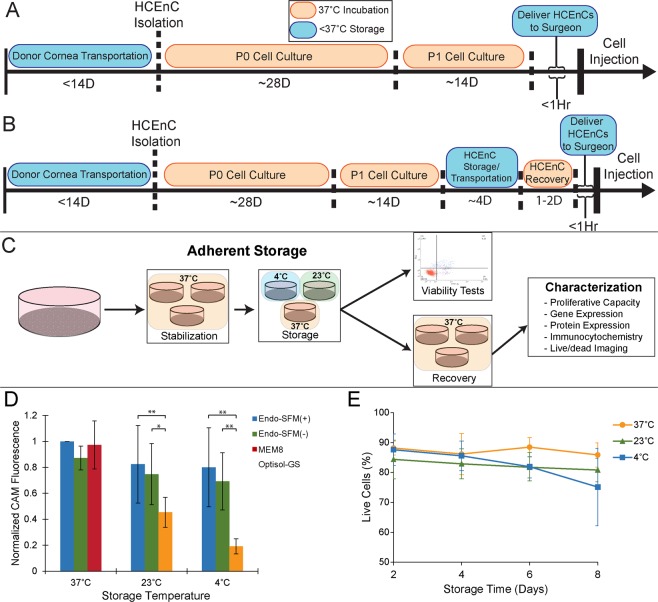
Figure 1.
Optimization of hypothermic storage protocol for corneal endothelial cells. (A) Current HCEnC culture protocols necessitate delivery of cells from the laboratory directly to the surgeon in a short time frame, while hypothermic storage (B) would create a window for storage/transport of cells. (C) To mimic an HCEnC-seeded scaffold, cells were initially stored as adherent monolayers in tissue culture dishes. Following storage, cells were processed directly to assess viability or returned to the incubator for 2 days of recovery before further analysis. (D) To determine the optimum storage medium, HCEnC viability was assessed with calcein AM (CAM) fluorescence after 2 days in storage, without any recovery at 37 °C. Multiple culture media were tested, including Endo-SFM with serum (Endo-SFM(+)) and without serum (Endo-SFM(-)) as well as Optisol-GS and an MEM-based organ culture medium with 8% serum. All fluorescence values were normalized to Endo-SFM(+) at 37 °C. Statistical significance was detected between storage in Optisol and Endo-SFM with and without serum at both 4 °C and 23 °C (*p < 0.05, **p < 0.01; n = 4). (E) Viability in Endo-SFM(+) over an extended hypothermic storage time was assessed using an Annexin V/propidium iodide flow cytometry assay. (n = 3).