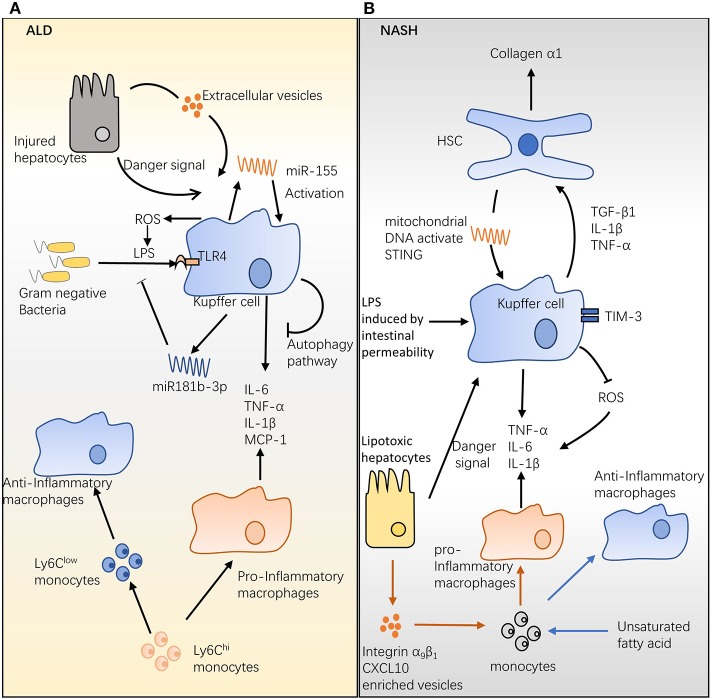
Figure 1.
Hepatic macrophages in alcoholic liver disease (ALD) and non-alcoholic steatohepatitis (NASH). (A) The role of hepatic macrophages in ALD. Chronic alcohol consumption disrupts the intestinal barrier, which increases the permeability of the gut and allows Gram-negative bacteria to migrate into the portal circulation. Lipopolysaccharide (LPS) expressed on Gram-negative bacteria activates Kupffer cells (KCs) and promotes interleukin (IL)-6, tumor necrosis factor (TNF)-α, IL-1β, and monocyte chemoattractant protein (MCP)-1 release. Hepatocytes injured by alcohol consumption activate KCs via danger signal and CD40-containing extracellular vesicles. Chronic alcohol ingestion induces microRNA (miR)-155 and miR181b-3p expression; the former activates KCs and promotes inflammatory production, while the latter regulates LPS-induced inflammation. The Ly6Chi monocyte can differentiate into pro-inflammatory and anti-inflammatory macrophages during ALD, and the ratio of these two populations may mediate ALD development. (B) The role of hepatic macrophages in NASH. High levels of LPS induced by increasing intestinal permeability and/or danger signal from lipotoxic hepatocytes stimulate KCs; activated KCs produce the survival signals, transforming growth factor β, IL-1β, and TNF-α, which stimulate hepatic stellate cells and increase generation of hepatic collagen α1, ultimately triggering fibrosis. Mitochondrial DNA from hepatocytes of high-fat diet (HFD)-fed mice activates KCs and promotes cytokine release, steatosis, and inflammation. Conversely, TIM-3 expressed on hepatic macrophages protects animals from HFD-induced NASH by inhibiting reactive oxygen species production. An HFD augments the infiltration of bone-marrow-derived monocytes into the liver and further differentiates them into protective anti-inflammatory macrophages.