Abstract
We report a case of a patient with pulmonary melioidosis which radiologically mirrored a bronchogenic carcinoma with mediastinal lymphadenopathy. Such findings were observed in a Computed Tomography (CT) scan (Thorax) and Positron Emission Tomography (PET) scan (Body) in a previously healthy 57-year-old man with no significant medical risk factors for melioidosis other than his previous exposures to soil during outfield military training in Thailand, 37 and 28 years ago. He presented with acute symptoms of dry cough, pleuritic pain and fever. A CT Scan thorax revealed a left lower lobe mass with left pleural and pericardial effusion and mediastinal lymph nodes. Similarly, the PET scan showed various fluorodeoxyglucose (FDG)-positron uptake in these areas. However the lung mass biopsy cultured Burkholderia pseudomallei concurrently with a Melioidosis serology titre of >1:1024. He responded to intravenous Meropenem followed by Co-Trimoxazole and Doxycycline over the course of 21 weeks, ultimately leading to the resolution of any significant radiological findings.
Keywords: Melioidosis, Pulmonary mass, Burkholderia pseudomallei
1. Introduction
Melioidosis was first described by Alfred Whitmore in 1912 when Whitmore and Krishnaswami reported a new glanders-like disease in postmortem examinations of 38 Rangoon vagrants [1]. Melioidosis is an enigmatic disease with protean clinical manifestations and has a remarkable propensity to affect any organ, thereby posing difficulties in establishing an accurate diagnosis. For this reason it has been dubbed as the great mimic of other diseases [2,3]. This report describes a case of an acute pulmonary melioidosis that was radiologically mistaken for a case of bronchogenic carcinoma.
2. Case Report
A previously healthy 57-year-old man presented to the emergency department with 2 weeks of history of chilling fever and dry cough associated with pleutic chest pain. Prior to this, he consulted his family doctor twice and was given two courses of antibiotics. However, his symptoms persisted.
Otherwise well, he has a 2 year history of hypertension and hyperlipidaemia, both well controlled with medications. The patient is married and is a non-smoker with insignificant travel history other than his national military service training in Thailand at age 20 and 29 years of age.
Initial vitals were stable with temperature of 37.6 °C, blood pressure of 142/76 mmHg, and SpO2 of 98% under room air. Physical examination revealed left sided pleural effusion affecting the lower one fifth of the left hemithorax. This was associated with reduced chest excursion and stony dullness on percussion as well as reduced vesicular breath sounds and vocal resonance on auscultation. Heart sounds were otherwise normal and no hepatosplenomegaly was noted.
Initial chest x-ray demonstrated left costophrenic angle blunting, left lower lobe consolidation and left hilar shadowing consistent with pleural effusion and pneumonia [Fig. 1]. Laboratory tests revealed significant leukocytosis of 18.13 × 10^9/L with 87.6% neutrophils and a CRP of 93.4mg/L with elevated liver enzymes, ALP of 184 U/L, SGOT 62 U/L, SGPT 66 U/L & GGT 141 U/L. Whereas, Mycoplasma IgM rapid test, Streptococcal urine antigen and respiratory multiplex panel PCR were all negative.
Fig. 1.
Left effusion, left hilar shadow and left lower lobe consolidation at initial presentation.
He was started on intravenous Ceftaroline for community acquired pneumonia & his fever settled on the second day of admission. However, his leucocyte count and CRP increased to 25.9 × 10^9/L and 225.60 mg/L respectively. In view of that, the antibiotic regimen was stepped up to Meropenem and Moxifloxacin on the fourth day of admission.
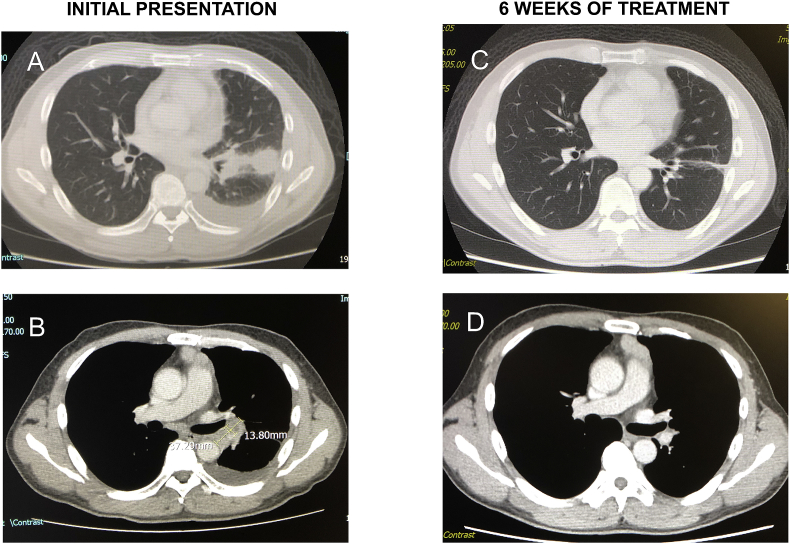
CT scan thorax on the fourth day of admission revealed a nodular mass measuring 3.3 × 2.7cm seen in the left lower lobe in contact with the lung pleura and it's fissure with internal necrosis [Fig. 2A]. There was also mediastinal adenopathy and pericardial effusion [Fig. 2B]. No abnormalities were noted on his ECG.
Fig. 2.
Comparing CT Scan Thorax at initial presentation & at 6 weeks of treatment. Fig. 2A: Left effusion, left lower lobe consolidation; Fig. 2B: Nodular mass at bifurcation of left main bronchus; Fig. 2C: CT Scan on 19/2/19 showing resolution of effusion and mass with residual scar; Fig. 2D: CT Scan on 19/2/19 showing resolution of effusion and left hilar node; At initial presentation - Multiple enlarged lymph nodes were seen throughout the mediastinum, most prominently in the prevascular space, anterior to the pulmonary artery bifurcation, measuring 4.5 × 3.2 cm. Two nodular masses were appreciated, one at the left main bronchus and the other measuring 3.3 × 2.7cm, more prominently seen in the left lower lobe in contact with the lung pleura and it's fissure with internal necrosis. Left pleural effusion was noted alongside pericardial effusion. At 6 weeks of treatment - Minimal lymph nodes in the precarinal, subcarinal and left hilar region were subcentimeter in size. The low density masses in the left lower lobe and left hilar region alongside with the initial pleural effusion have resolved with residual minor scarring and band atelectasis.
The patient underwent left pleural aspiration on the fifth day of admission, yielding 500 mls of straw-coloured fluid. Biochemical analysis of the pleural aspiration indicated the presence of an exudative effusion (LDH 699U/L). However cytology showed inflammatory yield with no malignant cells. Mycobacterium Tuberculosis complex DNA, AFB, fungal and bacterial smear/culture were all negative.
A PET scan conducted on the sixth day of admission demonstrated a hypermetabolic mass in the lower zone of the left lung with pleural thickening consistent with previous findings [Fig. 3A, B, C]. Incidental finding of an enlarged prostate gland was discovered, however no suspicious focal FDG activity was observed. We considered the possibility of a prostate malignancy given the raised PSA of 6.8 Ug/L, but ruled it out as the MRI prostate showed benign prostatic hyperplasia.
Fig. 3.
A: 3 × 2.5 cm mass at left lower lobe, SUX 7.9; B: 3 × 2.7 cm left hilar lymph node, SUX 7.8; C: 4 × 2.8 cm soft tissue density in anterior mediastinum, SUX 2.3. A hypermetabolic mass [Fig. 3A], in the lower zone of the left lung, abutting the adjacent pleura and left oblique fissure was noted with left pleural thickening. Several FDG avid lymph nodes were seen in the mediastinum and left hilar region, most significantly at the left hilar [Fig. 3B] compressing on the left lower lobe bronchus, while others at the pre-carinal region: 1 cm, SUX max 2.6, subcarinal region: 1.5 cm, SUX max 3.5, aorto-pulmonary window: 1.5 cm, SUX max 4.0. The prostate gland was enlarged but no suspicious focal FDG activity was observed.
After 3 days of Meropenem and Moxifloxacin, his leucocyte count dropped to 15.22 × 10^9/L and CRP reduced to 75.10 mg/L and procalcitonin was 0.09 ng/mL. Quantiferon TB Gold Plus was however indeterminate whereas his blood and sputum did not culture any organisms. Additionally he was cleared for Legionella and HIV. Tumour markers: CEA 0.6 μg/L, AFP <1.3 μg/L, CA 19–9 3.8 U/ml were otherwise normal.
Percutaneous lung biopsy was done on the seventh day of admission showing lung parenchymal tissue with a patchy infiltrate of acute and chronic inflammatory cells with areas of fibrosis and necrosis. No granuloma or malignancy were noted. The Ziehl-Neelsen stain for AFB, GMS stain for fungi and subsequent AFB and fungal cultures were all negative. Gram stain showed Gram negative rods, polymorphs +++, lymphocytes + and culture confirmed Burkholderia pseudomallei, sensitive to Co-Amoxiclav, Ceftazidime, Imipenem, Co-Trimoxazole and Tetracycline.
The Melioidosis serology reported on the twelfth day was positive with IHA titre of >1:1024. The Moxifloxacin was stopped after 9 days and Meropenem continued for a total of 14 days as he was clinically well with complete resolution of pleuritic chest pain. Subsequently discharged with Co-Trimoxazole and Doxycycline.
A repeated CT scan done at 6 weeks of treatment demonstrated gradual resolution of lymphadenopathy and pleural effusion [Fig. 2C and D].
3. Discussion
The infection is acquired by direct contact with contaminated soil and water through skin cuts and abrasions. It may enter the body through inhalation and ingestion. This infection is endemic in northern Australia and Southeast Asia, particularly in northeast Thailand. As such, Australia, Thailand and Singapore have the most reported cases of melioidosis worldwide [4]. In one Singapore study comprising one of the largest melioidosis patient cohorts based on national surveillance, it was found that the incidence of melioidosis has decreased in both bacteremia and pneumonia type of cases over the period 2003–2014 [5]. This was likely attributed to the enhanced environmental and water management systems with significant infrastructural improvements incorporated nationwide.
The only known potential risk for our patient was the prolonged soil exposure during his outfield military training in Singapore and Thailand 28–37 years ago. In latent infection, immunocompetent patients can clear the infection without showing any symptoms, with less than 5% of all melioidosis cases being activated after a period of latency [6]. There were no medical risk factors such as diabetes, chronic renal disease, chronic pulmonary condition or an immunocompromised state that might have predisposed him to reactivation. Although both the patient's CT scan (thorax) and PET scan (body) were suggestive of malignancy, antibiotics coverage was administered in view of the raised leucocyte count and CRP. Despite the provisional diagnosis of malignancy, the potentiality of co-infection had to be considered, therefore a culture was sent from the lung biopsy which indicated characteristic features of the Burkholderia pseudomallei organism. The result was verified at Singapore General Hospital. The lung mass culture yielded the only conclusive findings as cultures from other specimens such as blood, sputum and pleural fluid were repeatedly negative. Besides the pulmonary mass, the mediastinal lymph nodes and pericardial effusion [7] present were uncharacteristic of Melioidosis which inevitability contributed to a diagnostic dilemma.
Consent to publish
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Declaration of competing interest
The authors do not have any conflict of interests to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2020.101006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Whitmore A., Krishnaswami C.S. An account of the discovery of a hitherto undescribed infectious disease among the population of Rangoon. Indian Med. Gaz. 1912;47:262–267. [PMC free article] [PubMed] [Google Scholar]
- 2.Poe R.H., Vassallo C.L., Domm B.M., Bill M.D. Melioidosis: the remarkable imitator. Am. Rev. Respir. Dis. 1971;104:427–431. doi: 10.1164/arrd.1971.104.3.427. [DOI] [PubMed] [Google Scholar]
- 3.Yee K.C., Lee M.K., Chua C.T., Puthucheary S.D. Melioidosis, the great mimicker: a report of 10 cases from Malaysia. J. Trop. Med. Hyg. 1988;91:249–254. [PubMed] [Google Scholar]
- 4.Nasner-Posso K.M., Cruz-Calderon S., Montufar-Andrade F.E., Dance D.A., Rodriguez-Morales A.J. Human melioidosis reported by ProMED. Int. J. Infect. Dis. 2015;35:103–106. doi: 10.1016/j.ijid.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang L., Harris P.N.A., Seiler R.L., Ooi P.L., Cutter J., Goh K.T., Cook A.R., Fisher D., Chai L.Y.A. Melioidosis, Singapore, 2003-2014. Emerg. Infect. Dis. 2018;24(1) doi: 10.3201/eid2401.161449. www.cdc.gov/eid [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joost W., Wiersinga Harjeet, Virk S., Alfredo G Torres, Bart J Currie, 1 February Melioidosis. Nature Reviews Disease Primers. 2018;4(17107):17107. doi: 10.1038/nrdp.2017.107. PMID 29388572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Currie Bart J. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin. Respir. Crit. Care Med. February 2015;36(1):111–125. doi: 10.1055/s-0034-1398389. PMID 25643275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.