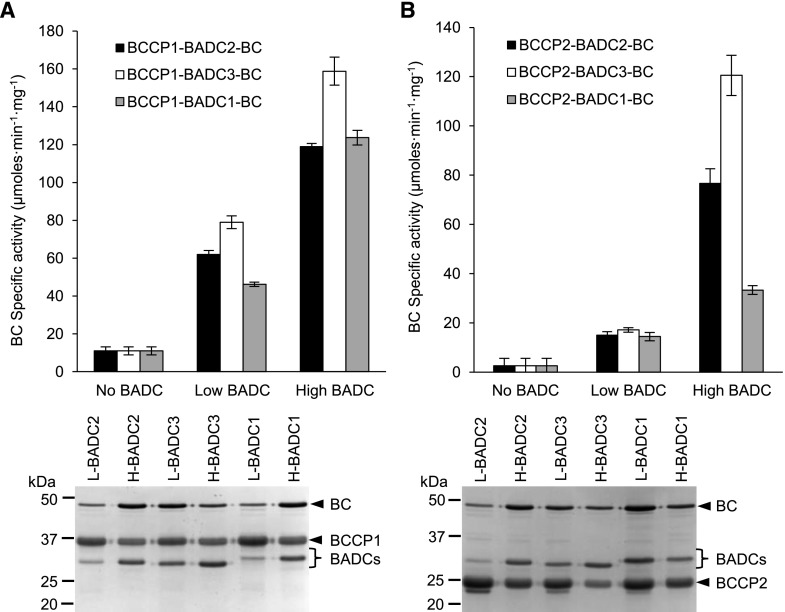
Figure 5.
BADC-facilitated assembly of BCCP–BADC–BC subcomplexes are activated in the ability to catalyze the bicarbonate-dependent hydrolysis of ATP in the first half-reaction of htACCase. Heterologous coexpression and copurification of either low- or high-level expression of individual BADCs with BC and BCCP subunits. Subcomplexes copurified by Ni-NTA affinity chromatography from E. coli protein extracts expressing His-tagged BCCP1 (A) or His-tagged BCCP2 (B) coexpressed with BC in the absence or presence of each BADC isoform expressed from a low-copy or high-copy number expression plasmid. Replicate assays formed at five different concentrations of BC protein, in the range of 0.5–10 µg per assay, determined the rate of bicarbonate-dependent appearance of ADP via the PK/LDH coupling reactions. L, proteins expressed from low copy number vectors; H, proteins expressed from high copy number expression vectors. Each data-point represents the mean ± se (n = 3), and the experiments were duplicated with analogous results.