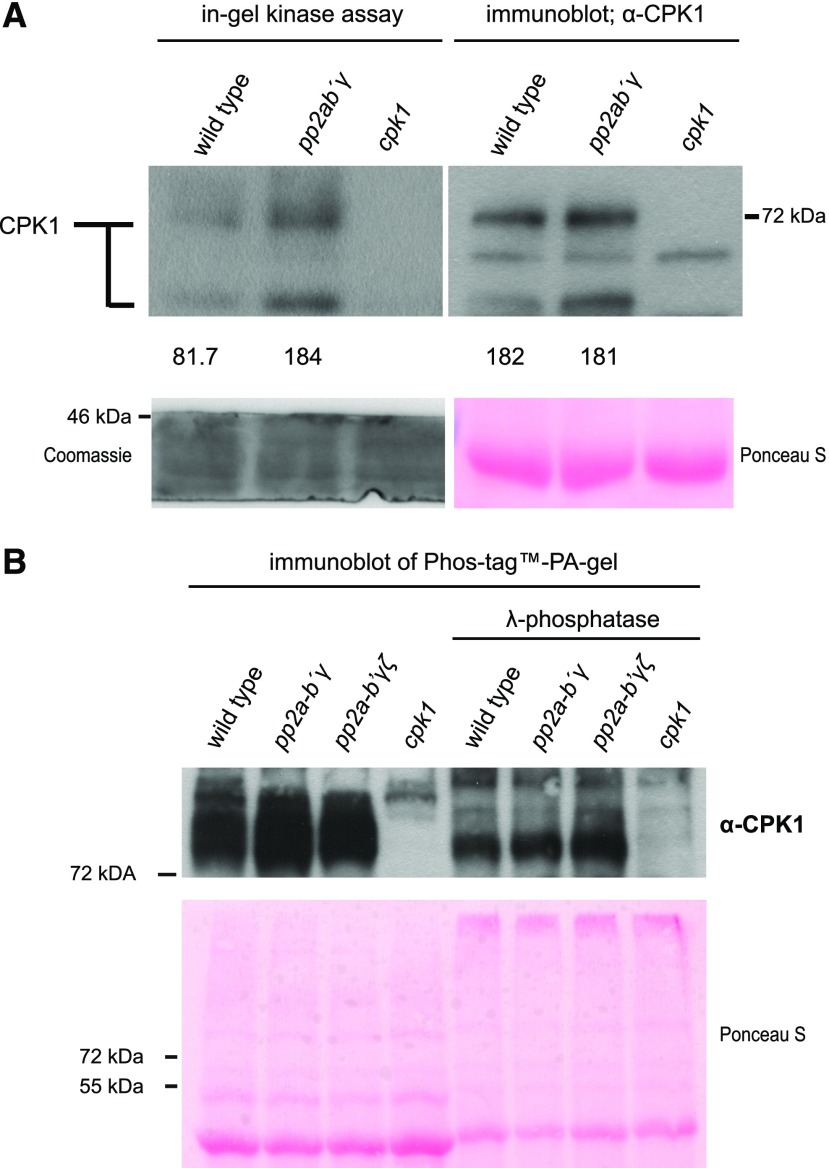
Figure 2.
Biochemical characterization of the PP2A-B′γ-CPK1 interaction. A, CPK1 in-gel kinase activity and protein abundance in total leaf extracts isolated from Arabidopsis wild type, pp2a-b′γ, and cpk1. CPK1 in-gel kinase activity (left) was assessed in the presence of 0.2 mm CaCl2 and histone type IIIS. Subsequently, the same samples were separated on SDS-gel electrophoresis and subjected to immunoblot analysis with a CPK1-specific antibody (right). Numbers (× 1,000) under the panels indicate intensity measurements for the uppermost band (Image Studio Lite; Li-Cor Biosciences). Loading controls are shown in the panels below the numbers; left: Coomassie Brilliant Blue R250-staining of the lower rim of the in-gel kinase SDS-PA gel; right: Ponceau S-staining of the large subunit of Rubisco on the PVDF-membrane. B, CPK1 immunoblot after separation of total leaf extracts from wild type, pp2a-b′γ, pp2a-b′γζ, and cpk1 on PhosTag PA gel. To assess the presence of phosphorylated residues among the high-molecular weight CPK1 bands, the samples were prepared in the presence and absence of lambda-phosphatase. Lower: Ponceau S-staining.