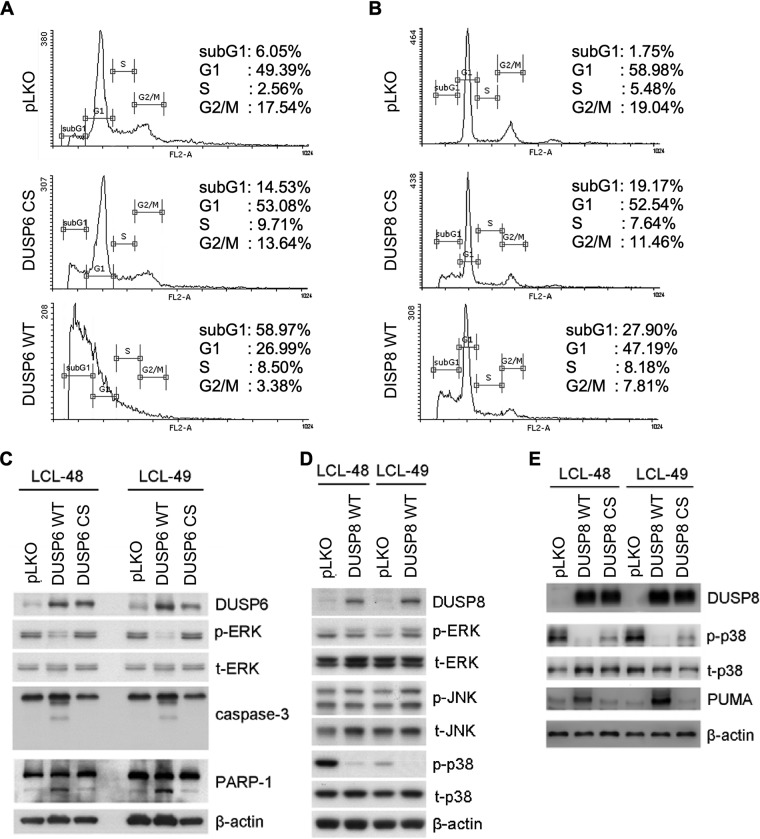
FIG 6.
Apoptosis caused by overexpression of DUSP6 and DUSP8. (A and B) LCLs were overexpressed with wild-type DUSP6 (DUSP6 WT), wild-type DUSP8 (DUSP8 WT) or their catalytic mutants (DUSP6 CS and DUSP8 CS). Selected cells underwent PI staining, and cell cycle patterns were analyzed with flow cytometry. Cells were selected with G418, and their lysates were analyzed by Western blotting. (C) Protein expression of DUSP6, phosphorylated ERK (p-ERK), total ERK (t-ERK), caspase-3, cleaved caspase-3, PARP-1, and cleaved PARP-1 was detected by immunoblotting. β-Actin served as an internal control. (D) Protein expression of DUSP8, phosphorylated p38 (p-p38), total p38 (t-p38), phosphorylated JNK (p-JNK), and total JNK (t-JNK) was detected. β-Actin served as an internal control. (E) Protein expression of DUSP8, phosphorylated p38 (p-p38), total p38 (t-p38), and PUMA was detected by Western blotting. β-Actin served as the internal control. DUSP6 and DUSP8 were transduced into LCLs through lentiviral infection, and the cells were selected with G418 for 5 days. (A and B) Selected cells underwent PI staining, and cell cycle patterns were analyzed with flow cytometry. (C to E) LCLs were overexpressed with wild-type DUSP6 (DUSP6 WT) or wild-type DUSP8 (DUSP8 WT) or with their catalytic mutants (DUSP6 CS and DUSP8 CS). Cells were selected with G418, and their lysates were analyzed by Western blotting. (C) Protein expression of DUSP6, phosphorylated ERK (p-ERK), total ERK (t-ERK), caspase-3, cleaved caspase-3, PARP-1, and cleaved PARP-1 was detected by immunoblotting. β-Actin served as an internal control. (D) Protein expression of WT DUSP8, phosphorylated ERK (p-ERK), total ERK (t-ERK), phosphorylated JNK (p-JNK), total JNK (t-JNK), phosphorylated p38 (p-p38), and total p38 (t-p38) was detected. β-Actin served as an internal control. (E) Protein expression of wild-type DUSP8, DUSP8 CS, phosphorylated p38 (p-p38), total p38 (t-p38), and PUMA was detected by Western blotting. β-Actin served as the internal control.