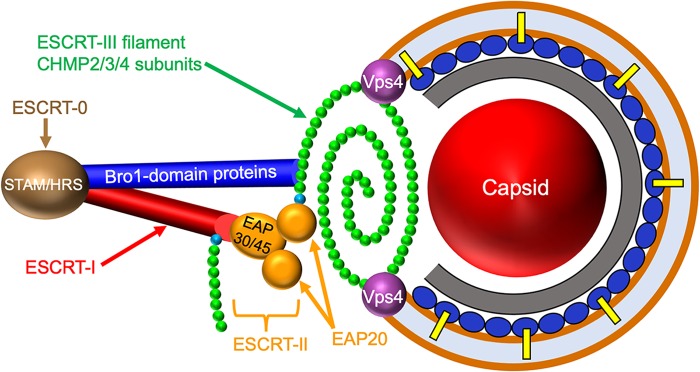
FIG 1.
Alphaherpesvirus envelopment and candidate ESCRT components. ESCRT complexes are shown on the left, with ESCRT-0 (composed of subunits STAM/HRS) in brown, ESCRT-I (composed of subunits TSG101/MVB12/VPS37/VPS28) in red, and ESCRT-II (EAP30/EAP45/EAP202) in yellow. The ESCRT-III filament is shown as a polymer of CHMP2/3/4 subunits (green) capped by nucleating CHMP6 subunits (cyan). The ESCRT-I and ESCRT-II complex EAP20 subunits can each nucleate filament assembly through recruitment of CHMP6. Members of the Bro1 family of proteins (dark-blue cylinder) are able to directly trigger CHMP4 polymerization. On the right is the generalized structure of an alphaherpesvirus cytoplasmic envelopment intermediate, showing the presumed relationship between the ESCRT-III filament and the lipid bilayer (brown line), the viral capsid (red sphere), the inner tegument (gray layer), and the outer tegument (dark-blue ovals), some of which interact with membrane-embedded envelope glycoproteins (yellow bars). Late in envelopment, ESCRT-III constricts to draw the membrane together, sealing the envelope and pinching the enveloped virus into the organellar lumen (light-blue space). Constriction and ESCRT-III disassembly are catalyzed by the ATPase VPS4 (purple spheres). The positioning of the ESCRT-0, -I, and -II complexes and the Bro1 proteins is not meant to necessarily imply they play roles in alphaherpesvirus envelopment.