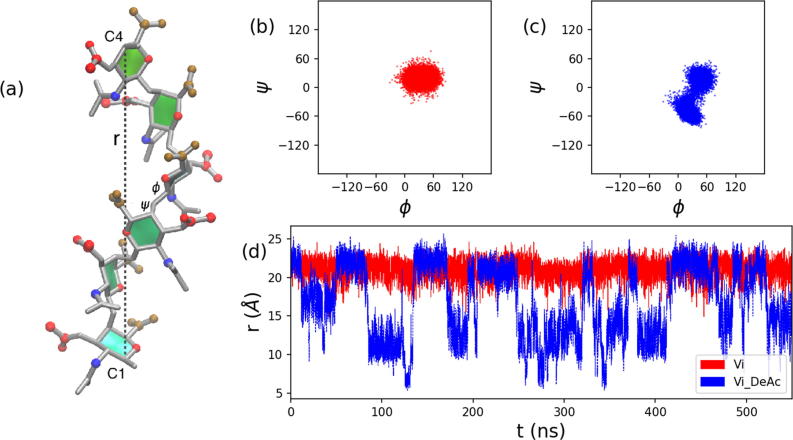
Fig. 8.
Comparison of the simulation dynamics in O-acetylated (red) and de-O-acetylated (blue) Vi polysaccharide. (a) An example conformation of O-acetylated Vi with the C1-C4 end-to-end distance, r, and the φ and ψ dihedral angles labeled. Atoms are highlighted as follows: N-acetyl,  ; O-acetyl,
; O-acetyl,  ; carboxyl,
; carboxyl,  . Simulation time series of the φ and ψ dihedral angles show differing conformations for the glycosidic linkage in (b) O-acetylated Vi and (c) de-O-acetylated Vi, with the de-O-acetylated strand rotating more freely (d). The same is true for the chain extension: the r end-to-end distance time series is relatively constant for O-acetylated Vi (
. Simulation time series of the φ and ψ dihedral angles show differing conformations for the glycosidic linkage in (b) O-acetylated Vi and (c) de-O-acetylated Vi, with the de-O-acetylated strand rotating more freely (d). The same is true for the chain extension: the r end-to-end distance time series is relatively constant for O-acetylated Vi ( ) and much more variable in de-O-acetylated Vi (
) and much more variable in de-O-acetylated Vi ( ).
).