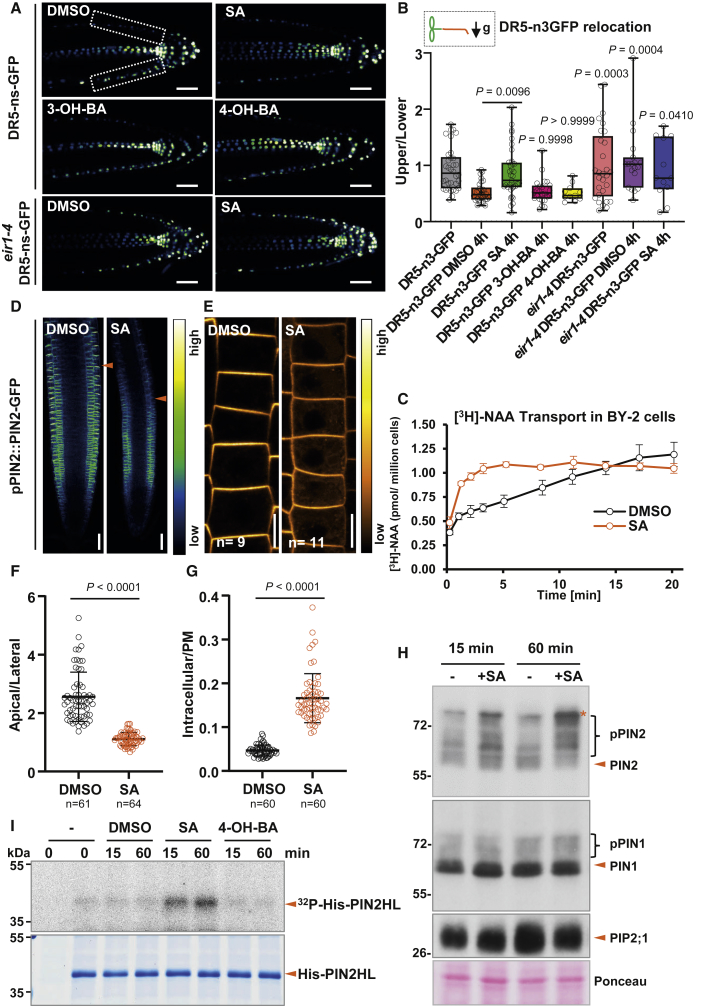
Figure 3.
SA Regulates Auxin Transport via Modulating PIN2 Phosphorylation
(A) SA inhibited the relocation of DR5-n3GFP. 5-day-old DR5v2 and eir1-4 DR5v2 seedlings were transferred to different plates with DMSO, 40 μM SA, 40 μM 3-OH-BA, or 40 μM 4-OH-BA, respectively, and then turned 90 degrees for gravistimulation. After 4 h, the roots were imaged by CLSM. The GFP channel (DR5-n3GFP) was shown. Scale bars, 10 μm.
(B) The ratio of fluorescence between the upper side and the lower side was measured, as shown in (A). n = 34, 30, 35, 24, 11, 29, 19, and 12, respectively. p values are calculated by a two-tailed t test, comparing different datasets with the DR5-n3-GFP DMSO control (t = 4 h), as shown with the horizontal line in the case of DR5-n3-GFP SA 4 h.
(C) SA treatment increased the accumulation of [3H]-NAA in tobacco BY-2 cells, suggesting a decrease in auxin export. DMSO and 200 μM SA were added to the cell culture and then the radioactivity inside of cells was measured at indicated time points after the addition of [3H]-NAA to the DMSO- and SA-treated cell cultures. n = 3.
(D–G) SA treatment impaired the polar localization and promoted the internalization of PIN2-GFP in root epidermis (D and E). pPIN2::PIN2-GFP seedlings were grown on plates with DMSO and 40 μM SA for 4 days and were then imaged by CLSM. Scale bars, 20 μm (D) and 10 μm (E), respectively. Arrowheads in (D) indicate the beginning of root transition zone.
(F) The intensity ratio of apical/lateral was measured by Fiji to assess PIN2 polarity.
(G) Quantification of the PIN2-GFP intensity ratio of intracellular/PM.
(F and G) Dots represent individual values, and lines indicate mean ± SD. p values are calculated by a two-tailed t test.
(H) SA treatment enhanced the phosphorylation of PIN2. Roots of 7-day seedlings were treated with DMSO or 40 μM SA for 15 min and 60 min and then analyzed by western blot with an anti-PIN2 antibody (upper panel). Phosphorylation of the multiple phosphorylation sites in PIN2 causes slower migrating species. The more highly phosphorylated, the slower the migration. The same membrane was stripped and detected by anti-PIN1 (second panel) and anti-PIP2;1 (third panel) antibodies, sequentially. The molecular weight (MW) of PIN2 and PIN1 is 69 and 67 kDa, respectively. For unknown reasons, PIN2 runs faster than expected, perhaps due to incomplete denaturing when heated only at 50°C. The shifted bands indicate the phosphorylated PIN proteins. Bottom panel: Ponceau staining is shown. Asterisk indicates partial contribution by a non-specific band (see also in Figure 4A).
(I) SA treatment increased the phosphorylation of His-PIN2-HL in plant extracts. Roots of 7-day seedlings were treated with DMSO or 40 μM SA for 15 min and 60 min, respectively, and then were subject to protein extraction. Crude plant extracts were incubated with recombinant His-PIN2-HL for 60 min with 32P-ATP and MgCl2. The first lane was without His-PIN2-HL as negative control. Reaction samples were analyzed by SDS-PAGE and the subsequent autoradiography. Upper panel: autoradiography is shown; lower panel: Coomassie Brilliant Blue (CBB) staining is shown.
See also Figures S2 and S3.