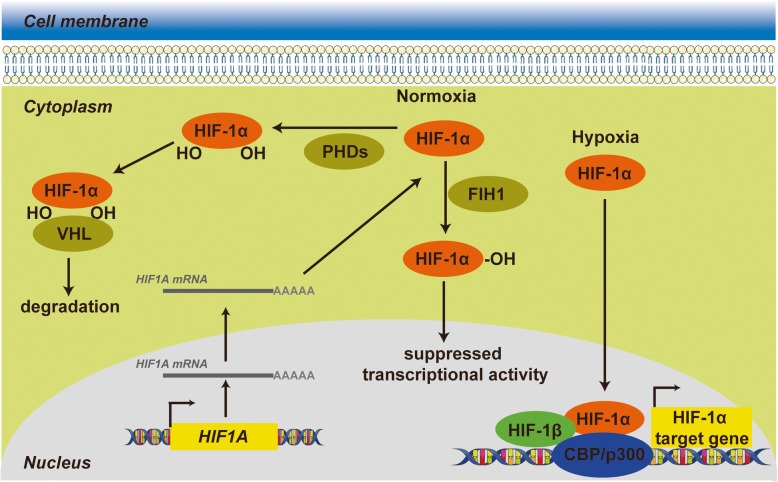
Fig. 1.
HIF-1α transcriptionally activates target genes in response to hypoxia. Under normoxia, HIF-1α is subjected to hydroxylation by PHDs and other prolyl hydroxylases. Hydroxylated HIF-1α is recognized by VHL proteins that target HIF-1α for subsequent ubiquitination and proteasomal degradation. In addition to regulation of the degradation of HIF-1α, the transcriptional activity of HIF-1α is regulated FIH1, which hydroxylates an asparagine residue of HIF-1α in its C-terminal transactivation domain and therefore blocks the interaction between HIF-1α and CBP/p300. During hypoxia, the hydroxylation reactions are diminished, resulting in HIF-1α accumulation and enhanced transcriptional activity, dimerization with HIF-1β, binding to target genes and activation of target genes through recruitment of CBP/p300 and formation of the transcription initiation complex.